This work reveals that Arabidopsis HY5, a well-characterized bZIP transcription factor involved in promoting photomorphogenesis, directly represses FHY1/FHL transcription in far-red light by negatively modulating the activities of two positive transcriptional activators FHY3 and FAR1.
Abstract
Phytochrome A (phyA) is the primary photoreceptor responsible for perceiving and mediating various responses to far-red light in Arabidopsis thaliana. FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL) are two small plant-specific proteins essential for light-regulated phyA nuclear accumulation and subsequent phyA signaling processes. FHY3 and its homolog FAR-RED IMPAIRED RESPONSE1 (FAR1) are two transposase-derived transcription factors that directly activate FHY1/FHL transcription and thus mediate subsequent phyA nuclear accumulation and responses. Here, we report that ELONGATED HYPOCOTYL5 (HY5), a well-characterized bZIP transcription factor involved in promoting photomorphogenesis, directly binds ACGT-containing elements a few base pairs away from the FHY3/FAR1 binding sites in the FHY1/FHL promoters. We demonstrate that HY5 physically interacts with FHY3/FAR1 through their respective DNA binding domains and negatively regulates FHY3/FAR1-activated FHY1/FHL expression under far-red light. Together, our data show that HY5 plays a role in negative feedback regulation of phyA signaling by attenuating FHY3/FAR1-activated FHY1/FHL expression, providing a mechanism for fine-tuning phyA signaling homeostasis.
INTRODUCTION
Phytochromes are red (R)/far-red (FR) light photoreceptors that play fundamental roles in photoperception of the light environment and the subsequent adaptation of plant growth and development (Quail et al., 1995; Whitelam and Devlin, 1997; Wang and Deng, 2003; Bae and Choi, 2008). There are five distinct phytochromes in Arabidopsis thaliana, designated phytochrome A (phyA) to phyE. PhyA is light-labile and is the primary photoreceptor responsible for perceiving and mediating various responses to FR light, whereas phyB-phyE are light-stable, and phyB is the predominant phytochrome regulating responses to R light (Sharrock and Quail, 1989; Somers et al., 1991; Nagatani et al., 1993; Parks and Quail, 1993; Reed et al., 1993; Whitelam et al., 1993). Phytochromes are synthesized in the cytosol in their inactive Pr form. Upon light irradiation, phytochromes are converted from the R light–absorbing Pr forms to the FR light–absorbing Pfr forms, and the Pfr forms of phytochromes (which are generally considered to be the biologically active forms) are translocated from the cytosol into the nucleus, triggering a signaling cascade that alters the expression of target genes and ultimately leads to the modulation of the biological responses (Sakamoto and Nagatani, 1996; Kircher et al., 2002; Quail, 2002; Jiao et al., 2007). Thus, light-regulated translocation of the photoreceptors from the cytosol into the nucleus is a key event in the phytochrome signaling cascade.
PhyA nuclear import is rapid and can be induced by either FR or R light; however, phyB nuclear import is relatively slow, only occurs in R light, and can be reversed by FR light (Kircher et al., 1999; Nagatani, 2004; Kevei et al., 2007; Fankhauser and Chen, 2008). The C-terminal half of phyB contains a putative nuclear localization signal (NLS), which is masked by the N-terminal half in darkness. Light triggers a conformational change in phyB, potentially unmasking the NLS and thus allowing its nuclear import (Chen et al., 2005; Fankhauser and Chen, 2008). By contrast, phyA does not contain any known NLS. Therefore, phyA translocation appears to depend on other components. Recently, it has been shown that two small plant-specific proteins, FAR-RED ELONGATED HYPOCOTYL1 (FHY1) and its homolog FHY1-LIKE (FHL), are essential for nuclear accumulation of light-activated phyA and subsequent light responses (Hiltbrunner et al., 2005, 2006; Rösler et al., 2007). A database search for FHY1/FHL homologs identified FHY1-like proteins in numerous plant species, and the only motifs conserved among these FHY1-like proteins are the NLS in their N-terminal region and the phyA binding domain at the C terminus, suggesting that the phyA nuclear import mechanism discovered in Arabidopsis might be conserved in higher plants (Genoud et al., 2008). Two transposase-derived transcription factors, FHY3 and FAR-RED IMPAIRED RESPONSE1 (FAR1), act together to activate directly the transcription of FHY1 and FHL, thus indirectly regulating phyA nuclear accumulation and phyA responses (Lin et al., 2007, 2008).
Genetic and molecular studies have led to the identification of numerous signaling intermediates that are either specific for individual photoreceptors or shared by multiple types of photoreceptors. Many of these intermediates are transcription factors or transcriptional regulators (for reviews, see Quail, 2002; Wang and Deng, 2003; Jiao et al., 2007). In addition to FHY3 and FAR1, a group of basic helix-loop-helix class transcription factors, also known as PHYTOCHROME INTERACTING FACTORS (PIFs), including PIF1, PIF3, PIF4, and PIF5, have been shown to bind photoactivated phytochromes directly and play central roles in phytochrome signaling networks (Ni et al., 1998; Huq and Quail, 2002; Kim et al., 2003; Huq et al., 2004; Khanna et al., 2004; Duek and Fankhauser, 2005; Castillon et al., 2007). Recent data showed that these PIF proteins accumulate in dark-grown seedlings and together act as constitutive repressors of photomorphogenesis, while upon light exposure, photoactivated phytochromes induce rapid phosphorylation and degradation of these transcription factors, allowing photomorphogenesis to begin (Bauer et al., 2004; Al-Sady et al., 2006; Shen et al., 2007; Leivar et al., 2008; Lorrain et al., 2008; Shen et al., 2008). ELONGATED HYPOCOTYL5 (HY5), a constitutively nuclear bZIP transcription factor, has been shown to function as a positive regulator of photomorphogenic development under a wide spectrum of wavelengths, including FR, R, blue (B), and UV-B, by binding directly to the promoters of a large number of light-responsive genes in vivo (Koornneef et al., 1980; Oyama, et al., 1997; Osterlund et al., 2000; Ulm et al., 2004; Lee et al., 2007). Multiple photoreceptors, including phytochromes and cryptochromes, promote the accumulation of HY5 under specific light conditions, possibly by reducing the nuclear abundance of CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1), an E3 ubiquitin ligase targeting HY5 for proteasome-mediated degradation in darkness (Osterlund and Deng, 1998; Osterlund et al., 2000). LONG HYPOCOTYL IN FAR-RED1 (HFR1) and LONG AFTER FAR-RED LIGHT1 (LAF1), atypical basic helix-loop-helix and R2R3-MYB transcription factors, respectively, were identified as positive regulators of phyA signaling (Fairchild et al., 2000; Fankhauser and Chory, 2000; Soh et al., 2000; Ballesteros, et al., 2001). Recently, it was shown that these two transcription factors interact with each other, and this interaction stabilizes both factors and enhances phyA photoresponses (Jang et al., 2007).
Previous studies showed that FHY1/FHL transcript levels were rapidly downregulated when dark-grown plants were exposed to FR light (Desnos et al., 2001; Lin et al., 2007), indicating that FHY1/FHL expression is subject to strict negative feedback regulation by phyA signaling, which is, however, poorly understood. Here, we show that the bZIP transcription factor HY5 acts in the feedback downregulation of FHY1/FHL expression by phyA signaling. We demonstrate that HY5 directly binds ACGT-containing elements (ACEs) in close proximity to the FHY3/FAR1 binding sites in the FHY1/FHL promoters. HY5 physically interacts with FHY3 and FAR1 and interferes with FHY3/FAR1 binding to the FHY1/FHL promoters. Thus, HY5 negatively regulates FHY1/FHL expression upon FR light exposure by modulating the activities of the transcriptional activators FHY3 and FAR1. Therefore, HY5 repression of FHY1/FHL expression likely represents a key mechanism in the feedback regulation of phyA signaling.
RESULTS
HY5 Directly Binds to the FHY1/FHL Promoters
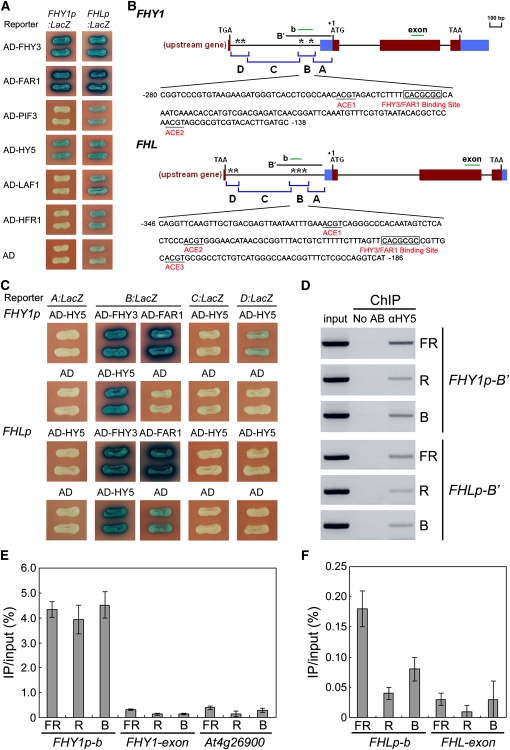
To investigate whether other transcription factors are involved in regulating FHY1/FHL expression, we performed yeast one-hybrid assays to test whether PIF3, HY5, LAF1, and HFR1, four transcription factors known to act in the phytochrome signaling pathways, bind to the FHY1 and FHL promoters. Our results show that of these four proteins, only HY5 binds directly to both promoters (FHY3 and FAR1 were included as positive controls) (Figure 1A), suggesting that HY5 may play a role in regulating FHY1/FHL expression.
Figure 1.
Transcription Factor HY5 Binds to the FHY1 and FHL Promoters in Yeast Cells and in Vivo.
(A) Yeast one-hybrid assays to test whether transcription factors PIF3, HY5, LAF1, and HFR1 bind to the FHY1 and FHL promoters. FHY3 and FAR1 were included as positive controls and empty vector expressing AD domain alone as negative controls. LacZ reporter gene expression (leading to blue color on the plates containing X-gal) was driven by the FHY1 or FHL promoter in yeast.
(B) Diagram of the promoter fragments of FHY1 and FHL and the sequences of B fragments of the FHY1 and FHL promoters. The exon-intron structure of FHL was based on the fact that FHL has an alternatively spliced transcript consisting of three exons and encoding a 201–amino acid isoform (GenBank accession number HM029245), in addition to the reported 181–amino acid FHL protein (Zeidler et al., 2001; Zhou et al., 2005). The genes upstream of FHY1 and FHL are At2g37680 and At5g02190, respectively. The adenine residue of the respective translational start codon (ATG) was assigned position +1, and the numbers flanking the sequences of the B fragments were counted based on this number. Asterisks indicate ACEs. A, B, C, and D indicate the corresponding promoter fragments used in yeast one-hybrid assays shown in (C). The black lines indicate the regions amplified by ChIP-PCR shown in (D). The short green lines depict the location of amplicons used for ChIP-qPCR shown in (E) and (F).
(C) Yeast one-hybrid assays showing that HY5 binds to the B fragments of the FHY1 and FHL promoters. FHY3 and FAR1 were included as positive controls, and empty vector expressing AD domain alone as negative controls.
(D) Representative results of the ChIP-PCR assays. Chromatin fragments (~500 bp) were prepared from 4-d-old wild-type Arabidopsis seedlings grown under continuous FR, R, and B light conditions, immunoprecipitated by the polyclonal HY5 antibodies, and the precipitated DNA PCR-amplified using the primer pairs indicated. Input, PCR reactions using the samples before immunoprecipitation. AB, antibody.
(E) and (F) qPCR analysis of the promoter (b fragments) and exon fragments of FHY1 and FHL in anti-HY5 ChIP assays. The 3′ untranslated region of At4g26900, a negative control used in a previous study (Lee et al., 2007), was used as a negative control in these assays. The ChIP values were normalized to their respective DNA inputs. Error bars represent sd of triplicate experiments.
As previous research indicated that HY5 binds ACEs (Chattopadhyay et al., 1998; Lee et al., 2007; Shin et al., 2007), we analyzed the distribution of ACEs in the FHY1 and FHL promoters. Interestingly, both promoters were found to harbor two short regions that contain ACE elements: one region very close to the stop codons of their respective upstream genes and the other region located ~150 to 300 bp upstream of their respective ATG start codons (Figure 1B). We then divided the FHY1/FHL promoters into four overlapping fragments, designated A, B, C, and D, in which the B and D fragments correspond to the two ACE-containing regions (Figure 1B), and generated yeast one-hybrid reporter constructs allowing the respective fragments to drive LacZ reporter gene expression. As expected, HY5 does not bind to the A or C fragments of either promoter, consistent with the fact that there are no ACEs in these fragments. Although HY5 weakly binds to the D fragment of the FHY1 promoter, it does not bind to the corresponding fragment of the FHL promoter (Figure 1C). By contrast, HY5 binds strongly to the B fragments of both promoters (Figure 1C), suggesting that the B fragments contain the major HY5 binding sites. As HY5 has a close homolog in the Arabidopsis genome, known as HY5 HOMOLOG (HYH), we tested whether HYH also binds to the FHY1 promoter. However, HYH failed to bind either the full-length or the B fragment of the FHY1 promoter (see Supplemental Figure 1 online), indicating that these two homologs target diverse genes. Interestingly, as the B fragments also contain FHY3/FAR1 binding sites (FBSs; Lin et al., 2007), FHY3 and FAR1 are able to bind to the B fragments of both promoters as well (Figure 1C). Collectively, our data show that HY5, FHY3, and FAR1 bind to the same fragments of the FHY1/FHL promoters.
A recent study using the chromatin immunoprecipitation (ChIP)-chip approach identified 3894 putative HY5 binding targets in the Arabidopsis genome (Lee et al., 2007), among which, FHY1 was listed as a potential HY5 binding target (see Supplemental Table 1 in Lee et al., 2007). To confirm this data experimentally and to examine whether HY5 binds to the FHL promoter in vivo, we performed ChIP assays using wild-type Arabidopsis seedlings. As HY5 protein accumulates mostly in light conditions (Osterlund et al., 2000; see Supplemental Figure 2 online), we then examined whether HY5 binds to the FHY1/FHL promoters in continuous FR, R, and B light. Our multiplex PCR and quantitative PCR (qPCR) both show that HY5 specifically binds to the FHY1 promoter in vivo in all three monochromatic light conditions (Figures 1D and 1E), compared with an exon fragment of FHY1 and the 3′ untranslated region of At4g26900, a negative control used in the previous ChIP-chip study. However, although our results also show that HY5 binds to the FHL promoter (Figures 1D and 1F), this binding seems much weaker than that to FHY1 as our qPCR data show that the recovery rate of an FHL promoter fragment in the FR ChIP sample is ~25-fold less than that of an FHY1 promoter fragment. Moreover, at least for the FHL promoter fragment, we observed a higher recovery rate in the FR sample than in the R and B samples (Figures 1D and 1F). Taken together, we conclude that HY5 binds to the FHY1 and FHL promoters in yeast cells and in vivo.
HY5 Binds the ACEs Closest to FBSs in the FHY1/FHL Promoters
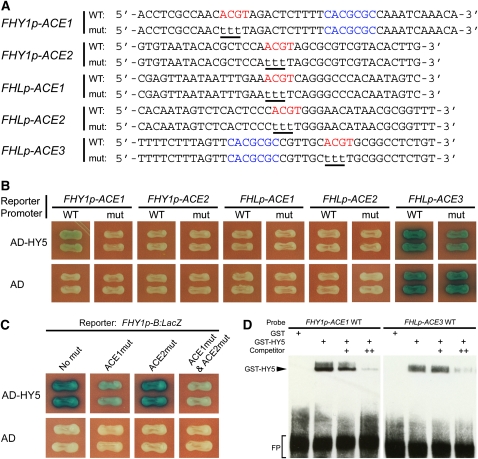
We next performed yeast one-hybrid assays to delineate the exact ACEs that are bound by HY5 in the B fragments of the FHY1/FHL promoters. As there are two and three ACEs in the B fragments of the FHY1 and FHL promoters, respectively, a total of 10 reporter constructs were generated to allow the respective wild-type subfragments and their corresponding mutants (mut; in which the respective ACGT was mutated to tttT) to drive LacZ reporter gene expression in yeast cells (Figure 2A). Our results show that for the two ACEs in the FHY1 promoter, HY5 binds the wild-type ACE1, but not its mutant, nor ACE2 in yeast cells (Figure 2B). We also mutated ACE1 and ACE2 in the context of the full B fragment of the FHY1 promoter to determine which ACE is the preferred binding site for HY5. Again, mutation of ACE1 almost abolished binding of HY5 to this promoter fragment, whereas mutation of ACE2 did not affect binding, confirming that HY5 primarily binds ACE1 in the B fragment of the FHY1 promoter (Figure 2C).
Figure 2.
HY5 Binds the ACEs Closest to the FHY3/FAR1 Binding Sites in the FHY1 and FHL Promoters.
(A) Diagram of the wild-type (WT) and mutant (mut) FHY1 and FHL subfragments used to drive LacZ reporter gene expression in yeast one-hybrid assays and as EMSA probes. Wild-type ACE elements are shown in red, and FBS motifs are shown in blue. Nucleotide substitutions in the mutant fragments are underlined.
(B) Yeast one-hybrid assays showing that HY5 binds the wild-type ACE1 of the FHY1 promoter but not ACE2 of the FHY1 promoter, or ACE1 or ACE2 of the FHL promoter.
(C) Yeast one-hybrid assays showing that ACE1 is the primary binding site for HY5 in the B fragment of the FHY1 promoter. The B fragment of the FHY1 promoter (sequence shown in Figure 1B) was mutated to abolish ACE1, ACE2, or both and used to drive LacZ reporter gene expression. In these assays, the respective ACGT was mutated to AaaT to facilitate mutagenesis reactions.
(D) EMSAs showing that GST-HY5 protein, but not GST by itself, specifically binds to the wild-type FHY1p-ACE1 and FHLp-ACE3 probes. The respective unlabeled probes (with the same sequence as the biotin-labeled probes) were used as competitors. FP, free probe.
For the three ACEs in the B fragment of the FHL promoter, our data show that HY5 does not bind to the wild-type subfragments of ACE1 or ACE2 (Figure 2B). However, for unknown reasons, the subfragments containing either wild-type or mutant ACE3 always induced strong LacZ reporter gene expression in yeast cells (Figure 2B). We therefore performed an electrophoretic mobility shift assay (EMSA) to test whether HY5 binds to the ACE3 subfragment in vitro. The ACE1 subfragment of the FHY1 promoter was also included in the assay as a positive control. Our data show that the GST-HY5 fusion protein (glutathione S-transferase fused with HY5), but not GST alone, bound the ACE1 subfragment of the FHY1 promoter and the ACE3 subfragment of the FHL promoter in vitro (Figure 2D), indicating that ACE3 is the HY5 binding site in the B fragment of the FHL promoter.
Interestingly, we noticed that ACE1 of the FHY1 promoter, and ACE3 of the FHL promoter, are the respective ACEs that are closest to FBSs in the FHY1/FHL promoters, and there are only 10 bp or less between these two cis-elements (Figure 1B). We therefore conclude that HY5 binds the ACEs closest to the FHY3/FAR1 binding sites in the FHY1 and FHL promoters.
HY5 Interacts with FHY3 and FAR1
As HY5 and FHY3/FAR1 bind cis-elements that are so close to each other in the FHY1/FHL promoters, it was intriguing to investigate whether these two types of transcription factors could physically interact with each other. To this end, we first performed yeast two-hybrid assays using bait vectors expressing either the full-length, N-terminal (harboring the COP1 interaction motif), or C-terminal (encoding the bZIP domain) domains of HY5 fused to the LexA DNA binding domain and prey vectors fusing the activation domain (AD) to the indicated Arabidopsis proteins (Figures 3A and 3B). Our data show that the full-length HY5 protein indeed interacts with FHY3 and FAR1, and the bZIP domain of HY5 is responsible for this interaction (AD-COP1 and AD-HY5 were included as positive controls for the N- and C-terminal domains of HY5, respectively) (Figure 3B).
Figure 3.
HY5 Interacts with FHY3 and FAR1.
(A) Schematic diagram of bait proteins (HY5, HY5N, and HY5C fused with LexA DNA binding domains).
(B) HY5C (corresponding to the bZIP domain of HY5) interacts with FHY3 and FAR1 in yeast cells. AD-COP1 and AD-HY5 were included as positive controls for interactions between HY5N and COP1 and the dimerization of bZIP domains, respectively.
(C) Schematic diagram of bait proteins (FHY3N, FHY3C1, FHY3C2, FAR1N, FAR1C1, and FAR1C2 fused with LexA DNA binding domains).
(D) Yeast two-hybrid assays showing that the DNA binding domains of FHY3 and FAR1 (FHY3N and FAR1N) interact with HY5.
(E) In vitro pull down of FHY3N and FAR1N with HY5. The 6×His-tagged N-terminal fragments of FHY3 and FAR1 pulled down with GST-HY5 or GST were detected by anti-His antibody. Input, 5% of the purified 6×His-tagged target proteins used in pull-down assays.
(F) Luciferase complementation imaging assays showing that HY5 interacts with FHY3N in plant cells. Tobacco leaves were transformed with the construct pairs HY5-NLuc/CLuc, HY5-NLuc/CLuc-FHY3N, and NLuc/CLuc-FHY3N. The leaves were observed for fluorescence imaging 3 d after the infiltration. The data shown are representative of three independent experiments, and error bars represent sd of four replicates. The middle panel shows an immunoblot for proteins isolated from tobacco leaves. Anti- full-length firefly LUC antibodies, which react with both the N- and C-terminal firefly LUC fragments, were used to detect the fusion proteins. The amount of protein loaded in each lane is indicated by Ponceau S staining of ribulose-1,5-bisphosphate carboxylase/oxygenase (bottom panel).
FHY3 and FAR1 were reported to contain three domains: an N-terminal C2H2 zinc finger domain, a central putative core transposase domain, and a C-terminal SWIM zinc finger domain (Lin et al., 2008). To define which of these domains of FHY3 and FAR1 interact with HY5, we fused them individually with LexA as indicated (Figure 3C) and then used these fusions as bait proteins in yeast two-hybrid assays. Unexpectedly, the SWIM zinc finger domain of FAR1 showed strong trans-activation activity in yeast cells (Figure 3D). Nevertheless, FHY3N and FAR1N also showed interaction with AD-HY5, allowing the conclusion that the C2H2 zinc finger motifs (i.e., the DNA binding domains of FHY3 and FAR1) may be responsible for interacting with HY5 (Figure 3D). To confirm this interaction further, we performed in vitro pull-down assays using GST-tagged HY5 and 6×His-tagged N-terminal domains of FHY3 and FAR1. Our data showed that GST-HY5, but not GST alone, was able to pull down the N-terminal domains of FHY3 and FAR1 (Figure 3E). Taken together, these data show that HY5 interacts with FHY3 and FAR1, and their DNA binding domains mediate their interactions.
To substantiate the physical interaction between HY5 and FHY3 in planta, we conducted firefly luciferase complementation imaging (LCI) assays (Chen et al., 2008) by transiently expressing HY5-NLuc and CLuc-FHY3N fusions in Nicotiana benthamiana leaf cells. As shown in Figure 3F, coexpression of HY5-NLuc and CLuc-FHY3N led to strong LUC activity. By contrast, HY5-NLuc or CLuc-FHY3N cotransformed with control vectors showed only background levels of LUC activity. These data support a physical interaction between HY5 and FHY3 in living plant cells.
HY5 Interferes with FHY3 for Binding to the FHY1 Promoter
As the HY5 binding site is only a few base pairs away from the FHY3 binding site in the FHY1 promoter, we next investigated how HY5 affects FHY3 binding to the FHY1 promoter by EMSAs using fusion proteins expressed in Escherichia coli. For FHY3, we used GST-tagged FHY3N (the first 200 amino acids of FHY3) and validated its biochemical activity by showing that it binds to the wild type but not the FBS-mutated FHY1 probe (Figure 4A). As GST-HY5 always migrates at a similar position as GST-FHY3N when they bind to the wild-type FHY1 probes (Figure 4A), we used GST-tagged HY5C (the amino acids 78 to 168 of HY5) instead in our assays. GST-HY5C also binds to the wild type but not the ACE1-mutated FHY1 probe and thus possesses the DNA binding activity of HY5 (Figure 4A).
Figure 4.
HY5 Interferes with FHY3 for Binding to the FHY1 Promoter.
(A) GST-FHY3N, GST-HY5, and GST-HY5C proteins, but not GST by itself, bind to the wild-type (WT) FHY1 promoter but not to the FBS (GST-FHY3N) and ACE (GST-HY5 and GST-HY5C) mutant (mut) probes. The sequences of FHY1p-ACE1 wild-type and mutant probes are shown in Figure 2A. The sequence of FHY1p-FBS mut probe is identical to that of the FHY1p-ACE1 wild-type probe except that the FBS motif (CACGCGC) was mutated to CAttttC. FP, free probe.
(B) Increasing amounts of GST-HY5C protein (lanes 7 to 9), but not GST (lanes 4 to 6), decrease the binding of GST-FHY3N to the wild-type FHY1 promoter. Interaction between HY5C and FHY3N prevents GST-FHY3N from binding to the promoter (lanes 10 to 12). The triangles in lanes 3 and 9 indicate a polymer of HY5C bound to the probe.
We then examined how HY5 affects FHY3 binding to the FHY1 promoter. As shown in Figure 4B, whereas increasing amounts of GST protein alone had no effect on GST-FHY3N binding to the promoter (lanes 4 to 6), increasing amounts of GST-HY5C protein obviously decreased the binding of GST-FHY3N to the wild-type FHY1 promoter (lanes 7 to 9). Then, we investigated how the interaction between HY5 and FHY3 affects FHY3 binding to the promoter using the ACE1-mutated probe to which HY5 could not bind. Notably, increasing amounts of GST-HY5C protein dramatically decreased the binding of GST-FHY3N to the FHY1 promoter even though GST-HY5C was not binding the probe (Figure 4B, lanes 10 to 12). Therefore, these data suggest that (1) increasing HY5 binding to the FHY1 promoter simultaneously decreases FHY3 binding to the promoter, and (2) the physical interaction between HY5 and FHY3 prevents FHY3 from binding to the FHY1 promoter.
HY5 Negatively Regulates FHY3/FAR1-Activated FHY1/FHL Transcription in Yeast and Plant Cells
To investigate how HY5 affects FHY3/FAR1-mediated FHY1/FHL transcription in yeast cells, we introduced another set of constructs into our yeast one-hybrid system. These pGAD-T7–based constructs were generated to express AD, HY5, or AD-HY5 fusion proteins in yeast cells (Figures 5A and 5B). As shown in Figure 5A, HY5 alone does not activate LacZ reporter gene expression, although it could bind to the FHY1 promoter (cf. 1 and 2), consistent with the previous report that HY5 lacks the transcriptional activation domain and is unable to activate transcription in yeast cells (Ang et al., 1998). However, addition of an activation domain to HY5 (AD-HY5) allows HY5 to activate LacZ reporter gene expression (Figure 5A, cf. 2 and 3). As mentioned above (Figures 1C and 2B), the B fragment of the FHL promoter (possibly due to the ACE3-containing sequence shown in Figure 2A) induced strong background expression of the LacZ reporter gene (Figure 5B, 1 and 2).
Figure 5.
HY5 Negatively Regulates FHY3/FAR1-Activated FHY1/FHL Transcription in Yeast and Plant Cells.
(A) and (B) Quantification of β-galactosidase activity in yeast cells harboring the FHY1p-B:LacZ (A) or FHLp-B:LacZ (B) reporter construct and coexpressing AD/AD-FHY3/AD-FAR1 and AD/HY5/AD-HY5 protein combinations shown on the left. Error bars represent sd (n = 4).
(C) Structure of the dual-luciferase reporter construct in which the firefly luciferase (LUC) reporter gene is driven by the wild type or ACE-mutated (both of the ACGT elements were mutated to AaaT) FHY1-B promoter fragment. The Renillia luciferase (REN) reporter gene is controlled by the constitutive 35S promoter. A 105-bp (−101 to +4) NOS minimal promoter (Puente et al., 1996) was inserted upstream of the LUC coding sequence to allow the promoter fragment to drive the LUC reporter gene transcription. Cauliflower mosaic virus terminator (Ter) and the T-DNA left border (LB) and right border (RB) are also indicated.
(D) Relative reporter activity in tobacco cells transiently transformed with the indicated effector and reporter constructs. FHY3 and HY5 are expressed by the 35S:FHY3 and 35S:HY5 effector plasmids (see Methods), respectively. Tobacco leaves were kept in white light for 4 d after infiltration. The relative LUC activities normalized to the REN activity are shown (LUC/REN). Error bars represent sd (n = 3).
[See online article for color version of this figure.]
We then examined how HY5 affects FHY3/FAR1-mediated reporter gene expression driven by the B fragments of the FHY1/FHL promoters. As shown in Figures 5A and 5B, AD-FHY3 and AD-FAR1 robustly activate LacZ reporter gene expression in yeast cells. However, coexpression of HY5 with AD-FHY3 or AD-FAR1 significantly decreases β-galactosidase activity (at least P < 0.05 for each compared group), implying that HY5 negatively regulates FHY3/FAR1-activated FHY1/FHL expression. Moreover, if an activation domain was added to HY5, the reporter gene expression was increased (Figures 5A and 5B), indicating that HY5 does occupy the FHY1/FHL promoters when it is coexpressed with FHY3/FAR1 in yeast cells.
We conducted a transient transcription assay in Nicotiana benthamiana leaves to study whether HY5 plays a similar regulatory role in plant cells. We generated dual-luciferase reporter constructs to allow the wild-type or ACE-mutated B fragment of the FHY1 promoter to drive LUC reporter gene expression (Figure 5C). As shown in Figure 5D, transiently expressed FHY3 acts as an activator of the FHY1 promoter. HY5 alone, however, does not show an obvious effect on transcription, consistent with its behavior in yeast cells. Coexpression of HY5 with FHY3 dramatically decreases the reporter gene expression to a similar level as HY5 alone, and this contrast is especially obvious when the ACEs in the FHY1p-B promoter fragment are mutated (Figure 5D).
Together with the EMSA assay data shown in Figure 4, we conclude that HY5 negatively regulates FHY1/FHL expression via two mechanisms. First, HY5 binds to the FHY1/FHL promoters, and as the binding sites of HY5 are in close proximity to those of FHY3/FAR1 in the FHY/FHL promoters, HY5’s occupation consequently decreases the accessibility of the promoters to FHY3/FAR1. Second, HY5’s interaction with FHY3/FAR1 may prevent them from binding to the FHY/FHL promoters. These two mechanisms are quite distinct from each other, but both seem important as HY5 could downregulate FHY1/FHL expression with or without its binding sites in the FHY1/FHL promoters provided FHY3/FAR1 are present (Figure 5). In either case, HY5 achieves its regulatory goal by modulating the activities of the transcriptional activators FHY3 and FAR1.
HY5 Negatively Regulates FHY1/FHL Transcript and FHY1 Protein Levels in Vivo
To confirm the role of HY5 in the regulation of FHY1/FHL expression in vivo, we compared the FHY1 and FHL transcript levels in wild-type and hy5 mutant seedlings by RNA gel blot and qRT-PCR analyses. Our data show that FHY1 and FHL transcript levels are notably elevated in hy5 mutant seedlings particularly under continuous FR light (Figures 6A to 6C), indicating that HY5 indeed negatively regulates FHY1 and FHL expression in Arabidopsis.
Figure 6.
HY5 Negatively Regulates FHY1 and FHL Transcript Levels in Vivo.
(A) RNA gel blot analysis showing FHY1 and FHL mRNA levels in 4-d-old wild-type (WT) and hy5 mutant seedlings grown in darkness (D) or continuous FR, R, and B light conditions. Ethidium bromide staining showing rRNA was used as the loading control.
(B) and (C) Real-time qRT-PCR analysis showing FHY1 and FHL transcript levels in wild-type and hy5 mutant seedlings. Error bars represent sd of triplicate experiments. *P < 0.05 and **P < 0.01 (Student’s t test) for the indicated pair of seedlings.
(D) and (E) Changes in FHY1 and FHL transcript levels in wild-type and hy5 mutant seedlings grown in darkness for 4 d and then transferred to FR light for various time periods. The expression levels in dark-grown seedlings were set as 1. Error bars represent sd of triplicate experiments.
(F) and (G) Immunoblots showing the changes of HY5 (F) and FHY3 (G) protein levels in wild-type seedlings grown in darkness for 4 d and then subjected to FR light treatment for various time periods. The mutant plants (hy5-215 and fhy3-1, respectively) were included as negative controls for immunoblots. Asterisk in (F) indicates a band cross-reacting with HY5 antibody. Anti-RPT5 was used as a sample loading control.
Previous reports showed that FHY1 and FHL transcript levels declined rapidly when the dark-grown wild-type seedlings were exposed to FR light (Desnos et al., 2001; Lin et al., 2007). We confirmed this pattern of FHY1/FHL downregulation using qRT-PCR (Figures 6D and 6E). To investigate the role of HY5 in this process, we examined the expression of FHY1 and FHL in hy5 mutant seedlings subjected to the same dark-to-FR light treatment. Intriguingly, expression of both FHY1 and FHL in hy5 mutants showed a lesser decrease compared with wild-type plants during the first 1 and 3 h, respectively, and then obviously increased during subsequent FR light treatment (Figures 6D and 6E), indicating that HY5 does play a major role in downregulating FHY1/FHL transcript levels in this FR light exposure treatment.
We then examined the levels of HY5 and FHY3 proteins in this time course to rule out the possibility that HY5 might downregulate FHY1/FHL transcript levels indirectly by downregulating FHY3 and FAR1 expression, as the expression of FHY3 and FAR1 displayed a pattern similar to that of FHY1 and FHL in this process (Lin et al., 2007). Our immunoblot data show that HY5 protein levels increased dramatically and continuously after the dark-grown wild-type seedlings were transferred to FR light (Figure 6F). By contrast, FHY3 protein levels showed a mild increase after 1 h of FR light exposure and then remained relatively stable in the subsequent FR light treatment, at least without showing an obvious decrease (Figure 6G). These data suggest that HY5 directly exerts its regulation on the FHY1 and FHL promoters, rather than indirectly through regulating expression of FHY3 and FAR1, although we cannot rule out the possibility that HY5 may regulate FHY3 and FAR1 transcript levels as well.
We further examined whether the abundance of FHY1 protein is correspondingly regulated by HY5. As reported in a recent study from our group (Shen et al., 2009), our FHY1 antibodies always recognize two endogenous FHY1 bands in immunoblots. Interestingly, only the smaller FHY1 band seems to be regulated by the hy5 mutation in different light conditions (Figure 7). Moreover, the abundance of the smaller FHY1 band was increased in hy5 mutants not only in continuous FR light but also in R and B light conditions, suggesting that HY5 may be involved in the posttranscriptional regulation of FHY1 as well.
Figure 7.
HY5 Negatively Regulates FHY1 Protein Levels.
FHY1 protein levels in 4-d-old fhy1, wild-type (WT), hy5, fhy3 far1, and phyA seedlings grown in darkness (D) or continuous FR, R, and B light conditions. The two arrowheads indicate two endogenous FHY1 bands in immunoblots recognized by our FHY1 antibodies (Shen et al., 2009). Anti-RPT5 was used as a sample loading control.
We also tested FHY1 protein accumulation in fhy3 far1 and phyA mutants. Consistent with the previous reports that FHY3 and FAR1 are key positive regulators of FHY1 expression (Desnos et al., 2001; Zhou et al., 2005; Lin et al., 2007), FHY1 protein level is severely attenuated in fhy3 far1 double mutants in darkness and all light conditions, comparable to that in fhy1 mutants (Figure 7). However, phyA only downregulates FHY1 protein level in light conditions (Figure 7), which might be achieved by direct phosphorylation of FHY1 by phyA under light conditions (Shen et al., 2009).
COP1 Positively Regulates FHY1/FHL Transcript Levels in Darkness
As HY5 was targeted for degradation by COP1 in darkness (Osterlund et al., 2000), and HY5 acts as a repressor of FHY1/FHL expression, we next examined whether COP1 positively regulates FHY1/FHL expression in darkness indirectly via HY5. To this end, we first confirmed that, as reported previously, HY5 protein level was extraordinarily elevated in cop1 mutants in darkness (Osterlund et al., 2000; see Supplemental Figure 3 online), while FHY3 protein level was not decreased by the cop1 mutation (data not shown). Then, we examined FHY1 and FHL transcript levels in dark-grown wild-type and cop1 mutant seedlings. Intriguingly, both FHY1 and FHL transcript levels in cop1 mutants were decreased to around 10% of that in the wild type (Figures 8A and 8B), suggesting that HY5 may play a major role in mediating COP1-regulated FHY1/FHL expression. Consistent with this finding, our previous study shows that COP1 is essential for FHY1 protein accumulation in darkness (Shen et al., 2005).
Figure 8.
COP1 Positively Regulates FHY1 and FHL Transcript Levels in Darkness.
Real-time qRT-PCR analysis showing that FHY1 (A) and FHL (B) transcript levels were markedly decreased in dark-grown cop1 mutants compared with wild-type (WT) plants. Error bars represent sd of triplicate experiments.
As the other COP/DET/FUS proteins, such as DET1, COP10, and the subunits of the COP9 signalosome, were shown to be required for degradation of HY5 in darkness (Osterlund et al., 2000), it is likely that these COP/DET/FUS proteins may positively regulate FHY1/FHL expression in darkness as well. Consistent with this assumption, our previous study also showed that these COP/DET/FUS proteins are required for normal accumulation of FHY1 protein in darkness (Shen et al., 2005).
HY5 Repression of FHY1 Expression Requires the Presence of FHY3 and FAR1
As discussed above, since HY5 lacks transcriptional repression activity in yeast and plant cells (Figure 5), it seems that HY5 represses FHY1/FHL expression by modulating the activities of their transcriptional activators FHY3 and FAR1. To test this hypothesis in vivo, we generated hy5 fhy3 far1 triple mutants to mutate both the positive regulators FHY3/FAR1 and the negative regulator HY5 of FHY1/FHL expression (see Supplemental Figure 4 online). We selected two independent triple mutant lines and confirmed that all three loci are homozygous in both lines (Figure 9A). qRT-PCR data show that in hy5 fhy3 far1 triple mutants, FHY1 transcript levels are similar to those in fhy3 far1 double mutants (Figure 9B), suggesting that HY5 repression of FHY1 expression requires the presence of its transcriptional activators FHY3 and FAR1 in vivo.
Figure 9.
HY5 Repression of FHY1 Expression Requires the Presence of FHY3 and FAR1.
(A) Detection of HY5 and FHY3 proteins in 4-d-old wild-type (Col and No-0 ecotypes), fhy3 far1, hy5, and two independent homozygous lines of hy5 fhy3 far1 triple mutant plants grown under continuous FR light. Anti-RPT5 was used as a sample loading control. The far1-2 mutation in the two lines of hy5 fhy3 far1 triple mutants was confirmed by directly sequencing the PCR products that contain the mutation (data not shown).
(B) Real-time qRT-PCR analysis showing FHY1 transcript levels in 4-d-old wild-type (Col and No-0 ecotypes), fhy1 fhl, fhy3 far1, phyA, hy5, and two independent homozygous lines of hy5 fhy3 far1 triple mutant plants grown under continuous FR light. Error bars represent sd of triplicate experiments.
DISCUSSION
FHY1 and FHL are two small proteins (202 and 201 amino acids, respectively) in Arabidopsis that were found to have homologs in both monocot and dicot plant species (Genoud et al., 2008). It was shown that FHY1 and FHL are required for nuclear accumulation of phyA since phyA is localized only in the cytosol of fhy1 fhl double mutants (Hiltbrunner et al., 2006; Rösler et al., 2007). This finding was greatly extended by a recent report that FHY3 and FAR1, two transposase-derived transcription factors, are the key activators of FHY1/FHL transcription and thus indirectly regulate phyA nuclear accumulation and phyA signaling (Lin et al., 2007). However, the mechanism by which FHY1/FHL expression is downregulated by the feedback regulation of phyA signaling remains unclear, although previous reports indicate that expression of FHY1/FHL, as well as FHY3/FAR1, are under this control (Desnos et al., 2001; Lin et al., 2007).
In this study, we show that the well-characterized Arabidopsis bZIP transcription factor HY5 directly represses FHY1/FHL expression in FR light, and, interestingly, this action of HY5 is accomplished by modulating the activities of the transcriptional activators FHY3 and FAR1. Because FHY1 and FHL are the key positive regulators of phyA signaling, the consequence of this action of HY5 may potentially attenuate phyA signaling. However, HY5 has been genetically defined as a positive regulator of phyA signaling, as mutations in HY5 cause a defect in the inhibition of hypocotyl elongation in continuous FR light (Oyama et al., 1997; Ang et al., 1998; see Supplemental Figure 4 online). In fact, our results may not be contradictory to the previous findings because this role of HY5 in downregulating FHY1/FHL transcript levels serves mainly in the feedback process of phyA signaling (i.e., phyA signaling has already been triggered and, thus, FHY1/FHL transcripts are not required at high levels), as it was shown that the accumulation of HY5 protein in FR light also requires phyA (Osterlund et al., 2000). Thus, once phyA is imported into the nucleus by FHY1 and FHL, phyA triggers a signaling cascade, and one consequence of this cascade is the accumulation of HY5, which acts to promote photomorphogenesis and downregulate FHY1/FHL transcript levels simultaneously (Figure 10). Therefore, our data suggest that HY5 plays dual roles in phyA signaling.
Figure 10.
A Working Model Depicting How HY5 Functions in the Feedback Regulation of phyA Signaling.
In the absence of light, FHY3 and FAR1 induce the expression of FHY1 and FHL in anticipation of the upcoming light signal. Accumulation of FHY1 and FHL proteins in dark-grown seedlings may serve to ensure rapid and sufficient phyA nuclear accumulation upon FR light exposure to jump start phyA signaling events in the nucleus. Upon light exposure, the Pfr form of phyA is imported into the nucleus by FHY1/FHL and thus triggers phyA signaling leading to multiple light responses, including the reduction of COP1 in the nucleus and accumulation of HY5 (Osterlund and Deng, 1998; Osterlund et al., 2000), and feedback regulation of FHY3 and FAR1 transcript levels (Lin et al., 2007). HY5 plays dual roles in phyA signaling: promoting photomorphogenesis and downregulating FHY1/FHL transcript levels by modulating the activities of the transcriptional activators FHY3 and FAR1. FHY3 and FHY1 (indicated by larger letters) are the more predominant players in the phyA signaling process compared with their respective homologs FAR1 and FHL. Pr, R-absorbing form of phyA (inactive); Pfr, FR-absorbing form of phyA (active). Arrow, positive regulation; bar, negative regulation.
Because FHY1 and FHL regulate phyA nuclear accumulation, and HY5 negatively regulates FHY1 (and possibly FHL) protein levels (Figure 7), it is reasonable to propose that HY5 might negatively regulate phyA nuclear accumulation. However, we failed to detect any difference in nuclear phyA levels between FR-grown wild-type and hy5 mutant seedlings in our nuclear fractionation assays (data not shown). This result may be explained by two possibilities. First, nuclear phyA levels may be tightly controlled, as one molecular event after phyA is imported into the nucleus by FHY1/FHL is the degradation of the photoreceptor (Seo et al., 2004; Saijo et al., 2008; Debrieux and Fankhauser, 2010). Thus, the excess phyA proteins imported into the nucleus by the increased levels of FHY1/FHL proteins may be degraded rapidly. Second, overaccumulation of FHY1/FHL may affect mainly the kinetics of phyA nuclear accumulation, rather than the steady state levels of nuclear phyA, whereas nuclear fractionation assays only roughly detect the steady state levels of nuclear phyA. So the question whether HY5 regulates phyA nuclear accumulation will require further investigation and better techniques.
We show that HY5 acts as a repressor of FHY1/FHL expression. However, HY5 itself does not show any transcriptional activity in yeast and plant cells (Figure 5). Our data suggest that HY5 achieves its regulatory effects by modulating the activities of FHY3/FAR1, two activators of FHY1/FHL expression. This conclusion is also supported by the examination of FHY1 expression in hy5 fhy3 far1 triple mutants (Figure 9). In another report, HY5 was shown to be necessary for normal circadian expression of the Lhcb genes via interaction with CCA1 (Andronis et al., 2008). Consistent with these findings, a recent study demonstrates that HY5 binding to the target gene promoters is not sufficient for transcriptional regulation, implying that HY5 may need other cofactors to regulate target gene expression (Lee et al., 2007). Moreover, a transcription factor protein microarray study discovered 20 transcription factor candidates that may interact with HY5 (Gong et al., 2008). Thus, the discovery of more HY5-interacting cofactors and elucidation of more regulatory modes of HY5 may help in understanding how HY5 implements its hierarchical role in promoting photomorphogenesis.
In summary, our work reveals an interesting new role of HY5 in the feedback regulation of phyA signaling. Taken together with the previous reports, the feedback regulation of phyA signaling is achieved by at least four distinct mechanisms: negative regulation of FHY1/FHL expression by HY5 shown in this study, FHY3/FAR1 repression by phyA signaling (Lin et al., 2007), direct phosphorylation of FHY1 by phyA and subsequent FHY1 degradation (Shen et al., 2005, 2009), and phosphorylation and subsequent degradation of phyA itself (Saijo et al., 2008). The multiple layers of feedback regulation imply a complicated and delicate fine-tuned control of phyA signaling, thus allowing plants to respond quickly, appropriately, and precisely to their dynamic light environment.
METHODS
Plant Materials and Growth Conditions
The wild-type Arabidopsis thaliana used in this study is of the Columbia (Col) ecotype, unless otherwise indicated. The phyA-211 (Reed et al., 1994), fhy1-3 (Zeidler et al., 2001), fhy1-3 fhl-1 (Rösler et al., 2007), hy5-215 (Oyama et al., 1997), fhy3-1 (Whitelam et al., 1993), and cop1-4 (McNellis et al., 1994) mutants are of the Col ecotype, and the fhy3-4 (Wang and Deng, 2002) and fhy3-4 far1-2 (Lin et al., 2007) mutants are of the No-0 ecotype and have been described previously. The hy5 fhy3 far1 triple mutant was constructed by crossing hy5-215 and fhy3-4 far1-2 mutants. The mutation hy5-ks50 (Oyama et al., 1997) was introduced into the Col background by genetic backcrossing (Lee et al., 2007). The growth conditions and light sources were as described previously (Shen et al., 2005).
Plasmid Construction
The FHY1p:LacZ and FHLp:LacZ reporter constructs were described previously (Lin et al., 2007). To generate FHY1p-A:LacZ, FHY1p-B:LacZ, FHY1p-C:LacZ, FHY1p-D:LacZ, FHLp-A:LacZ, FHLp-B:LacZ, FHLp-C:LacZ, and FHLp-D:LacZ reporter constructs, the promoter fragments were amplified by PCR using FHY1p:LacZ and FHLp:LacZ constructs as the templates and the respective pairs of primers (see Supplemental Table 1 online), and then cloned into the EcoRI-XhoI sites of the pLacZi2μ vector (Lin et al., 2007), respectively. To generate various LacZ reporter genes driven by the wild-type and mutant subfragments of the FHY1 and FHL promoters shown in Figure 2B, oligonucleotides were synthesized as two complementary oligo primers with an EcoRI site overhang at the 5′ end and an XhoI site overhang at the 3′ end (see Supplemental Table 1 online). The oligo primers were annealed, and the double-stranded oligonucleotides were ligated into the EcoRI-XhoI sites of the pLacZi2μ vector, producing FHY1p-ACE1WT:LacZ, FHY1p-ACE2WT:LacZ, FHLp-ACE1WT:LacZ, FHLp-ACE2WT:LacZ, FHLp-ACE3WT:LacZ, FHY1p-ACE1mut:LacZ, FHY1p-ACE2mut:LacZ, FHLp-ACE1mut:LacZ, FHLp-ACE2mut:LacZ, and FHLp-ACE3mut:LacZ, respectively.
To mutate the respective ACEs in the B fragment of the FHY1 promoter, the FHY1p-B:LacZ reporter plasmid (described above) was used as the template using the QuikChange site-directed mutagenesis kit (Stratagene) and the primers shown in Supplemental Table 1 online according to the manufacturer’s instructions. To generate the FHY1p-B(ACEm):LacZ reporter construct in which both of the ACGT elements were mutated into AaaT, two rounds of mutagenesis reactions were performed, with each round introducing one mutation into the promoter.
The AD-FHY3, AD-FAR1, and AD-COP1 constructs were described previously (Ang et al., 1998; Wang and Deng, 2002). To generate AD-PIF3, AD-HY5, AD-LAF1, AD-HFR1, and AD-HYH, the full-length coding sequences of PIF3, HY5, LAF1, HFR1, and HYH were amplified by PCR with the respective pairs of primers (see Supplemental Table 1 online) and then cloned into the EcoRI-XhoI sites of the pB42AD vector (Clontech), respectively. To generate pGAD-T7 constructs for expressing HY5 and AD-HY5 fusion proteins in yeast cells, the full-length coding sequence of HY5 was amplified by PCR with the respective pairs of primers (see Supplemental Table 1 online) and then cloned into the KpnI-XhoI and EcoRI-XhoI sites of the pGAD-T7 vector (Clontech), respectively.
The LexA-HY5, LexA-HY5N, and LexA-HY5C constructs were described previously (Ang et al., 1998). To generate LexA-FHY3N, LexA-FHY3C1, and LexA-FHY3C2, the fragments were amplified by PCR with the respective pairs of primers (see Supplemental Table 1 online) and then cloned into the BamHI-SalI sites of the pLexA vector (Clontech), respectively. To generate LexA-FAR1N, LexA-FAR1C1, and LexA-FAR1C2, the respective PCR fragments were cloned into the BamHI-XhoI sites of the pLexA vector, respectively.
The GST-HY5 and GST-FHY3N constructs were described previously (Ang et al., 1998; Lin et al., 2007). To generate GST-HY5C, the PCR fragment was cloned into the BamHI-XhoI sites of the pGEX-4T-1 vector (Amersham Biosciences). To generate 6×His-FHY3N and 6×His-FAR1N, the PCR fragments were cloned into the BamHI-SalI and BamHI-XhoI sites of the pET-28a vector (Novagen), respectively.
To generate HY5-NLuc, a BamHI-SalI PCR fragment of full-length HY5 was cloned into the corresponding sites of the vector 35S:NLuc (Chen et al., 2008). To generate CLuc-FHY3N, a KpnI-SalI PCR fragment of FHY3N (first 260 amino acids) was cloned into the corresponding sites of the vector 35S:CLuc (Chen et al., 2008).
To generate the 35S:FHY3 vector, a BamHI-SalI fragment containing the full-length coding sequence of FHY3 was released from LexA-FHY3 (Wang and Deng, 2002), and then the released fragment was inserted into the BamHI-SalI sites of the pSPYNE-35S vector (Walter et al., 2004). To generate the 35S:HY5 vector, a BamHI-SalI PCR fragment of full-length HY5 was cloned into the corresponding sites of the pSPYNE-35S vector.
To generate the FHY1p-B:LUC and FHY1p-B(ACEm):LUC reporter constructs, a PstI-BamHI PCR fragment containing the 105 bp (−101 to +4) NOS minimal promoter was amplified from NOS101-GUS (Puente et al., 1996) and inserted into the corresponding sites of pGreenII 0800-LUC (Hellens et al., 2005), resulting in miniPro:LUC vector. Then, the B fragment of the FHY1 promoter was amplified by PCR using FHY1p-B:LacZ and FHY1p-B(ACEm):LacZ constructs (described above) as the templates and cloned into the KpnI-XhoI sites of miniPro:LUC to produce the FHY1p-B:LUC and FHY1p-B(ACEm):LUC vectors, respectively.
All of the primers used to generate the above-mentioned constructs are listed in Supplemental Table 1 online, and all of the constructs were confirmed by sequencing prior to usage in various assays.
Yeast Assays
For yeast one-hybrid assays, plasmids for AD fusions were cotransformed with the LacZ reporter genes driven by various FHY1 and FHL promoter fragments into the yeast strain EGY48; for yeast two-hybrid assays, the respective combinations of LexA and AD fusion plasmids were cotransformed into the yeast strain EGY48, which already contains the reporter plasmid p8op:LacZ (Clontech). Transformants were grown on proper dropout plates containing X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for blue color development. Yeast transformation and liquid assay were conducted as described in the Yeast Protocols Handbook (Clontech).
Immunoblotting
For anti-FHY3 immunoblots, Arabidopsis seedlings were ground to a fine powder and total proteins were eluted in 2× SDS loading buffer. For all the other immunoblots, Arabidopsis seedlings were homogenized in an extraction buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.1% Tween 20, 1 mM PMSF, 40 μM MG132, and 1× complete protease inhibitor cocktail (Roche). Immunoblotting was performed as previously described (Shen et al., 2005).
Primary antibodies used in this study include anti-FHY1 (Shen et al., 2005), anti-HY5 (Osterlund et al., 2000), anti-FHY3 (Saijo et al., 2008), and anti-RPT5 (Kwok et al., 1999) antibodies.
ChIP
Wild-type seedlings grown under continuous FR, R, and B light conditions for 4 d were used for ChIP assays following the procedure described previously (Lee et al., 2007). Briefly, 2 g of seedlings grown under the indicated light conditions were first cross-linked with 1% formaldehyde under vacuum. The samples were ground to powder in liquid nitrogen, and the chromatin complexes were isolated and sonicated and then incubated with polyclonal HY5 antibodies (Osterlund et al., 2000). The precipitated DNA was recovered and analyzed by PCR methods using the primers in Supplemental Table 1 online. Real-time qPCR analysis was performed using the respective pair of primers and Power SYBR Green PCR Master Mix (Applied Biosystems) with a Bio-Rad CFX96 real-time PCR detection system. PCR reactions were performed in triplicate for each sample, and the ChIP values were normalized to their respective DNA input values.
Preparation of Recombinant Proteins
All constructs were transformed into Escherichia coli BL21 (DE3) cells that were treated with isopropyl-β-d-thiogalactoside to induce fusion protein expression. The GST fusion proteins were purified with Glutathione Sepharose 4B beads (Amersham Biosciences), and the 6×His-fusion proteins were purified with nickel-nitrilotriacetic acid beads (Qiagen).
EMSA
EMSAs were performed using biotin-labeled probes and the Lightshift Chemiluminescent EMSA kit (Pierce). The sequences of the complementary oligonucleotides used to generate the biotin-labeled and unlabeled probes are shown in Supplemental Table 1 online. Briefly, 0.5 μg of GST or GST fusion proteins were incubated together with biotin-labeled probes in 20-μL reaction mixtures containing 10 mM Tris-HCl, 150 mM KCl, 1 mM DTT, 50 ng/μL poly (dI-dC), 2.5% glycerol, 0.05% Nonidet P-40, 100 μM ZnCl2, and 0.5 μg/μL BSA for 20 min at room temperature and separated on 6% native polyacrylamide gels in Tris-glycine buffer. For the competition assays shown in Figure 4B, 0.5, 1, and 2 μg of GST or GST-HY5C proteins were used, respectively. The labeled probes were detected according to the instructions provided with the EMSA kit.
RNA Gel Blot Analysis and Real-Time qRT-PCR
Total RNA was extracted from Arabidopsis seedlings using the RNeasy plant mini kit (Qiagen). For RNA gel blot analysis, 15 μg of total RNA were loaded per lane and blotting was performed as described previously (Martínez et al., 2004). Fragments of FHY1 and FHL used for probe labeling were generated by PCR, and the primers are shown in Supplemental Table 1 online.
For real-time qRT-PCR, cDNAs were synthesized from 2 μg total RNA using SuperScript II first-strand cDNA synthesis system (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed as described above. PCR reactions were performed in triplicate for each sample, and the expression levels were normalized to that of a ubiquitin gene.
In Vitro Pull-Down Assay
For in vitro binding, 2 μg of purified recombinant bait proteins (GST-HY5 and GST) and 2 μg of prey proteins (6×His-FHY3N and 6×His-FAR1N) were added to 1 mL of binding buffer containing 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 0.6% Triton X-100. After incubation at 4°C for 2 h, Glutathione Sepharose 4B beads (Amersham Biosciences) were then added and incubated for a further 1 h. After washing three times with the binding buffer, pulled-down proteins were eluted in 2× SDS loading buffer at 95°C for 10 min, separated on 10% SDS-PAGE gels, and detected by immunoblotting using anti-His antibody (Qiagen).
LCI Assay
Transient LCI assays in Nicotiana benthamiana were performed as described previously (Chen et al., 2008). Briefly, Agrobacterium tumefaciens (strain GV2260) bacteria containing indicated constructs were infiltrated into young but fully expanded leaves of the 7-week-old N. benthamiana plants using a needleless syringe. After infiltration, plants were grown under 16-h light/dark for 3 d, and luciferase signals were then viewed in an IVIS Spectrum imaging system (Caliper LifeSciences) and quantified with the Living Image 4.0 software. To confirm the expression of the NLuc and CLuc fusion proteins, total protein was extracted from equal amounts of tobacco leaves and subjected to immunoblot analysis with anti- firefly LUC antibodies (Sigma-Aldrich), which react with both the N- and C-terminal firefly LUC fragments. The amount of protein loaded in each lane is indicated by Ponceau S staining of ribulose-1,5-bisphosphate carboxylase/oxygenase.
Transient Transcription Dual-Luciferase Assay
Transient dual-luciferase assay in N. benthamiana was performed as described previously (Hellens et al., 2005). After infiltration, plants were left under continuous white light for 4 d, and then leaf samples were collected. Firefly luciferase and Renillia luciferase were assayed using the dual luciferase assay reagents (Promega) and were performed essentially as previously described (Liu et al., 2008). Briefly, leaf discs (1 to 2 cm in diameter) were excised, ground in liquid nitrogen, and homogenized in 100 μL of the Passive Lysis Buffer. Eight microliters of this crude extract was mixed with 40 μL of Luciferase Assay Buffer, and the firefly luciferase (LUC) activity was measured using a GLOMAX 20/20 luminometer (Promega). Forty microliters of Stop and Glow Buffer was then added to the reaction, and the Renillia luciferase (REN) activity was measured. Three biological repeats were measured for each sample.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: FHY1 (At2g37678), FHL (At5g02200), FHY3 (At3g22170), FAR1 (At4g15090), HY5 (At5g11260), PIF3 (At1g09530), LAF1 (At4g25560), HFR1 (At1g02340), COP1 (At2g32950), and PHYA (At1g09570).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. HYH Does Not Bind to the FHY1 Promoter.
Supplemental Figure 2. HY5 Protein Levels in Wild-Type Seedlings Grown in Different Light Conditions.
Supplemental Figure 3. HY5 Protein Levels in Wild-Type and cop1 Mutant Seedlings in Darkness.
Supplemental Figure 4. Phenotypes of the Various Arabidopsis Mutants Defective in phyA Signaling.
Supplemental Table 1. Summary of Primers Used in This Study.
Supplementary Material
Acknowledgments
We thank Mathias Zeidler for fhy1-3 and fhy1-3 fhl-1 seeds, Jian-Min Zhou for 35S:NLuc and 35S:CLuc plasmids, Chentao Lin for pGreenII 0800-LUC plasmid, and Tian Xu for kindly sharing their imaging system. We also thank Shangwei Zhong and Jeffery Q. Shen for their suggestions on the project and William Terzaghi and Hongwei Guo for critical comments on the manuscript. This work was supported by a National Institutes of Health grant (GM47850) to X.W.D. and in part by a National Science Foundation award (MCB-1004808) to H.W. Studies conducted at Peking University were supported by grants from the Ministry of Science and Technology of China (2009DFB30030) and the Ministry of Agriculture of China (2009ZX08012-021B).
References
- Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446 [DOI] [PubMed] [Google Scholar]
- Andronis C., Barak S., Knowles S.M., Sugano S., Tobin E.M. (2008). The clock protein CCA1 and the bZIP transcription factor HY5 physically interact to regulate gene expression in Arabidopsis. Mol. Plant 1: 58–67 [DOI] [PubMed] [Google Scholar]
- Ang L.H., Chattopadhyay S., Wei N., Oyama T., Okada K., Batschauer A., Deng X.W. (1998). Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell 1: 213–222 [DOI] [PubMed] [Google Scholar]
- Bae G., Choi G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59: 281–311 [DOI] [PubMed] [Google Scholar]
- Ballesteros M.L., Bolle C., Lois L.M., Moore J.M., Vielle-Calzada J.P., Grossniklaus U., Chua N.H. (2001). LAF1, a MYB transcription activator for phytochrome A signaling. Genes Dev. 15: 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adám E., Fejes E., Schäfer E., Nagy F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillon A., Shen H., Huq E. (2007). Phytochrome interacting factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 12: 514–521 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S., Ang L.H., Puente P., Deng X.W., Wei N. (1998). Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10: 673–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zou Y., Shang Y., Lin H., Wang Y., Cai R., Tang X., Zhou J.M. (2008). Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146: 368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Tao Y., Lim J., Shaw A., Chory J. (2005). Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15: 637–642 [DOI] [PubMed] [Google Scholar]
- Debrieux D., Fankhauser C. (2010). Light-induced degradation of phyA is promoted by transfer of the photoreceptor into the nucleus. Plant Mol. Biol. 73: 687–695 [DOI] [PubMed] [Google Scholar]
- Desnos T., Puente P., Whitelam G.C., Harberd N.P. (2001). FHY1: A phytochrome A-specific signal transducer. Genes Dev. 15: 2980–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duek P.D., Fankhauser C. (2005). bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci. 10: 51–54 [DOI] [PubMed] [Google Scholar]
- Fairchild C.D., Schumaker M.A., Quail P.H. (2000). HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14: 2377–2391 [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C., Chen M. (2008). Transposing phytochrome into the nucleus. Trends Plant Sci. 13: 596–601 [DOI] [PubMed] [Google Scholar]
- Fankhauser C., Chory J. (2000). RSF1, an Arabidopsis locus implicated in phytochrome A signaling. Plant Physiol. 124: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud T., Schweizer F., Tscheuschler A., Debrieux D., Casal J.J., Schäfer E., Hiltbrunner A., Fankhauser C. (2008). FHY1 mediates nuclear import of the light-activated phytochrome A photoreceptor. PLoS Genet. 4: e1000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W., He K., Covington M., Dinesh-Kumar S.P., Snyder M., Harmer S.L., Zhu Y.X., Deng X.W. (2008). The development of protein microarrays and their applications in DNA-protein and protein-protein interaction analyses of Arabidopsis transcription factors. Mol. Plant 1: 27–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltbrunner A., Tscheuschler A., Viczián A., Kunkel T., Kircher S., Schäfer E. (2006). FHY1 and FHL act together to mediate nuclear accumulation of the phytochrome A photoreceptor. Plant Cell Physiol. 47: 1023–1034 [DOI] [PubMed] [Google Scholar]
- Hiltbrunner A., Viczián A., Bury E., Tscheuschler A., Kircher S., Tóth R., Honsberger A., Nagy F., Fankhauser C., Schäfer E. (2005). Nuclear accumulation of the phytochrome A photoreceptor requires FHY1. Curr. Biol. 15: 2125–2130 [DOI] [PubMed] [Google Scholar]
- Huq E., Al-Sady B., Hudson M., Kim C., Apel K., Quail P.H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305: 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E., Quail P.H. (2002). PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 21: 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I.C., Yang S.W., Yang J.Y., Chua N.H. (2007). Independent and interdependent functions of LAF1 and HFR1 in phytochrome A signaling. Genes Dev. 21: 2100–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Lau O.S., Deng X.W. (2007). Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 8: 217–230 [DOI] [PubMed] [Google Scholar]
- Kevei E., Schafer E., Nagy F. (2007). Light-regulated nucleo-cytoplasmic partitioning of phytochromes. J. Exp. Bot. 58: 3113–3124 [DOI] [PubMed] [Google Scholar]
- Khanna R., Huq E., Kikis E.A., Al-Sady B., Lanzatella C., Quail P.H. (2004). A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16: 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Yi H., Choi G., Shin B., Song P.S., Choi G. (2003). Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15: 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S., Gil P., Kozma-Bognár L., Fejes E., Speth V., Husselstein-Muller T., Bauer D., Adám E., Schäfer E., Nagy F. (2002). Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S., Kozma-Bognar L., Kim L., Adam E., Harter K., Schafer E., Nagy F. (1999). Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11: 1445–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Rolff E., Spruit C.J.P. (1980). Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z. Pflanzenphysiol. 100: 147–160 [Google Scholar]
- Kwok S.F., Staub J.M., Deng X.W. (1999). Characterization of two subunits of Arabidopsis 19S proteasome regulatory complex and its possible interaction with the COP9 complex. J. Mol. Biol. 285: 85–95 [DOI] [PubMed] [Google Scholar]
- Lee J., He K., Stolc V., Lee H., Figueroa P., Gao Y., Tongprasit W., Zhao H., Lee I., Deng X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19: 731–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P., Monte E., Oka Y., Liu T., Carle C., Castillon A., Huq E., Quail P.H. (2008). Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr. Biol. 18: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Ding L., Casola C., Ripoll D.R., Feschotte C., Wang H. (2007). Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Teng Y., Park H.J., Ding L., Black C., Fang P., Wang H. (2008). Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction. Plant Physiol. 148: 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yu X., Li K., Klejnot J., Yang H., Lisiero D., Lin C. (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Martínez C., Pons E., Prats G., León J. (2004). Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 37: 209–217 [DOI] [PubMed] [Google Scholar]
- McNellis T.W., von Arnim A.G., Araki T., Komeda Y., Miséra S., Deng X.W. (1994). Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell 6: 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A. (2004). Light-regulated nuclear localization of phytochromes. Curr. Opin. Plant Biol. 7: 708–711 [DOI] [PubMed] [Google Scholar]
- Nagatani A., Reed J.W., Chory J. (1993). Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M., Tepperman J.M., Quail P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95: 657–667 [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Deng X.W. (1998). Multiple photoreceptors mediate the light-induced reduction of GUS-COP1 from Arabidopsis hypocotyl nuclei. Plant J. 16: 201–208 [DOI] [PubMed] [Google Scholar]
- Osterlund M.T., Hardtke C.S., Wei N., Deng X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405: 462–466 [DOI] [PubMed] [Google Scholar]
- Oyama T., Shimura Y., Okada K. (1997). The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11: 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.M., Quail P.H. (1993). hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell 5: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente P., Wei N., Deng X.W. (1996). Combinatorial interplay of promoter elements constitutes the minimal determinants for light and developmental control of gene expression in Arabidopsis. EMBO J. 15: 3732–3743 [PMC free article] [PubMed] [Google Scholar]
- Quail P.H. (2002). Phytochrome photosensory signalling networks. Nat. Rev. Mol. Cell Biol. 3: 85–93 [DOI] [PubMed] [Google Scholar]
- Quail P.H., Boylan M.T., Parks B.M., Short T.W., Xu Y., Wagner D. (1995). Phytochromes: Photosensory perception and signal transduction. Science 268: 675–680 [DOI] [PubMed] [Google Scholar]
- Reed J.W., Nagatani A., Elich T.D., Fagan M., Chory J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler J., Klein I., Zeidler M. (2007). Arabidopsis fhl/fhy1 double mutant reveals a distinct cytoplasmic action of phytochrome A. Proc. Natl. Acad. Sci. USA 104: 10737–10742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Zhu D., Li J., Rubio V., Zhou Z., Shen Y., Hoecker U., Wang H., Deng X.W. (2008). Arabidopsis COP1/SPA1 complex and FHY1/FHY3 associate with distinct phosphorylated forms of phytochrome A in balancing light signaling. Mol. Cell 31: 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Nagatani A. (1996). Nuclear localization activity of phytochrome B. Plant J. 10: 859–868 [DOI] [PubMed] [Google Scholar]
- Seo H.S., Watanabe E., Tokutomi S., Nagatani A., Chua N.H. (2004). Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R.A., Quail P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3: 1745–1757 [DOI] [PubMed] [Google Scholar]
- Shen H., Zhu L., Castillon A., Majee M., Downie B., Huq E. (2008). Light-induced phosphorylation and degradation of the negative regulator PHYTOCHROME-INTERACTING FACTOR1 from Arabidopsis depend upon its direct physical interactions with photoactivated phytochromes. Plant Cell 20: 1586–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Feng S., Ma L., Lin R., Qu L.J., Chen Z., Wang H., Deng X.W. (2005). Arabidopsis FHY1 protein stability is regulated by light via phytochrome A and 26S proteasome. Plant Physiol. 139: 1234–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Khanna R., Carle C.M., Quail P.H. (2007). Phytochrome induces rapid PIF5 phosphorylation and degradation in response to red-light activation. Plant Physiol. 145: 1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Zhou Z., Feng S., Li J., Tan-Wilson A., Qu L.J., Wang H., Deng X.W. (2009). Phytochrome A mediates rapid red light-induced phosphorylation of Arabidopsis FAR-RED ELONGATED HYPOCOTYL1 in a low fluence response. Plant Cell 21: 494–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Park E., Choi G. (2007). PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 49: 981–994 [DOI] [PubMed] [Google Scholar]
- Soh M.S., Kim Y.M., Han S.J., Song P.S. (2000). REP1, a basic helix-loop-helix protein, is required for a branch pathway of phytochrome A signaling in arabidopsis. Plant Cell 12: 2061–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D.E., Sharrock R.A., Tepperman J.M., Quail P.H. (1991). The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell 3: 1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm R., Baumann A., Oravecz A., Máté Z., Adám E., Oakeley E.J., Schäfer E., Nagy F. (2004). Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 101: 1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang H., Deng X.W. (2002). Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J. 21: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Deng X.W. (2003). Dissecting the phytochrome A-dependent signaling network in higher plants. Trends Plant Sci. 8: 172–178 [DOI] [PubMed] [Google Scholar]
- Whitelam G., Devlin P. (1997). Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 20: 752–758 [Google Scholar]
- Whitelam G.C., Johnson E., Peng J., Carol P., Anderson M.L., Cowl J.S., Harberd N.P. (1993). Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler M., Bolle C., Chua N.H. (2001). The phytochrome A specific signaling component PAT3 is a positive regulator of Arabidopsis photomorphogenesis. Plant Cell Physiol. 42: 1193–1200 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Hare P.D., Yang S.W., Zeidler M., Huang L.F., Chua N.H. (2005). FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1. Plant J. 43: 356–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.