Abstract
Context
Hypertonic fluids restore cerebral perfusion with reduced cerebral edema and modulate inflammatory response to reduce subsequent neuronal injury and thus have potential benefit in resuscitation of patients with traumatic brain injury (TBI).
Objective
To determine whether out-of-hospital administration of hypertonic fluids improves neurologic outcome following severe TBI.
Design, Setting, and Participants
Multicenter, double-blind, randomized, placebo-controlled clinical trial involving 114 North American emergency medical services agencies within the Resuscitation Outcomes Consortium, conducted between May 2006 and May 2009 among patients 15 years or older with blunt trauma and a prehospital Glasgow Coma Scale score of 8 or less who did not meet criteria for hypovolemic shock. Planned enrollment was 2122 patients.
Intervention
A single 250-mL bolus of 7.5% saline/6% dextran 70 (hypertonic saline/dextran), 7.5% saline (hypertonic saline), or 0.9% saline (normal saline) initiated in the out-of-hospital setting.
Main Outcome Measure
Six-month neurologic outcome based on the Extended Glasgow Outcome Scale (GOSE) (dichotomized as >4 or ≤4).
Results
The study was terminated by the data and safety monitoring board after randomization of 1331 patients, having met prespecified futility criteria. Among the 1282 patients enrolled, 6-month outcomes data were available for 1087 (85%). Baseline characteristics of the groups were equivalent. There was no difference in 6-month neurologic outcome among groups with regard to proportions of patients with severe TBI (GOSE ≤4) (hypertonic saline/dextran vs normal saline: 53.7% vs 51.5%; difference, 2.2% [95% CI, −4.5% to 9.0%]; hypertonic saline vs normal saline: 54.3% vs 51.5%; difference, 2.9% [95% CI, −4.0% to 9.7%]; P=.67). There were no statistically significant differences in distribution of GOSE category or Disability Rating Score by treatment group. Survival at 28 days was 74.3% with hypertonic saline/dextran, 75.7% with hypertonic saline, and 75.1% with normal saline (P=.88).
Conclusion
Among patients with severe TBI not in hypovolemic shock, initial resuscitation with either hypertonic saline or hypertonic saline/dextran, compared with normal saline, did not result in superior 6-month neurologic outcome or survival.
Trial Registration
clinicaltrials.gov Identifier: NCT00316004
Traumatic brain injury (TBI) is the leading cause of death following blunt trauma, and survivors often sustain severe disability. TBI is responsible for the greatest number of potential years of life lost from any cause and carries the highest burden on loss of quality-adjusted life-years among survivors.1 The primary injury to the brain occurs at the time of impact; however, subsequent compromise of cerebral perfusion can lead to an ischemic insult that extends the primary injury, creating a secondary brain injury.2
Current therapy following severe TBI is focused on minimizing secondary injury by supporting systemic perfusion and reducing intracranial pressure (ICP). Intravenous fluid resuscitation currently begins in the out-of-hospital setting; however, therapy for management of cerebral edema is often delayed until after hospital arrival.
Hypertonic fluids have been shown to decrease ICP and improve cerebral perfusion pressure in animal models and patients with severe TBI.3-6 Hypertonic saline has also been shown to have beneficial vasoregulatory, immunomodulatory, and neurochemical effects on the injured brain.7 Previous trials have suggested that early administration of hypertonic fluids to patients with severe TBI may improve survival, but no large definitive trials have been reported and the effects on neurologic outcome are not known.8,9 Furthermore, all studies have focused on patients with severe TBI and hypovolemic shock; thus, the effect of early administration of hypertonic fluids for patients with severe TBI in the absence of hypovolemic shock is also not known.
We hypothesized that administration of hypertonic fluids as early as possible after severe TBI in patients without hemorrhagic shock would result in improved 6-month neurologic outcome.
METHODS
This study was conducted by the Resuscitation Outcomes Consortium, a multicenter clinical trial network including 11 regional clinical centers in the United States and Canada. The trial involved 114 emergency medical services agencies within the catchment area served by the consortium.10 Two trials with 2 distinct patient cohorts, one for hypovolemic shock and the other for TBI, were conducted simultaneously using the same intervention.
This report describes the outcome of the TBI cohort. This was a double-blind, 3-group, randomized controlled clinical trial comparing a 250-mL bolus of 7.5% saline (hypertonic saline) vs 7.5% saline/6% dextran 70 (hypertonic saline/dextran) vs 0.9% saline (normal saline) as the initial resuscitation fluid administered to injured patients with suspected severe TBI in the out-of-hospital setting. This dose of hypertonic saline and hypertonic saline/dextran was selected because it was the dose used in all previous prehospital trials and thus had a proven safety record. Previous studies suggested that the expected serum sodium level on admission would be 145 to 155 mEq/L. Details of the initial study design have been published.11
Patient Population
Patients were included in the TBI cohort based on the following: blunt mechanism of injury, age 15 years or older, Glasgow Coma Scale (GCS) score of 8 or less, and ineligibility for enrollment in the hemorrhagic shock cohort. The hemorrhagic shock cohort included all patients with systolic blood pressure of 70 mm Hg or less or of 71 to 90 mm Hg with a concomitant heart rate of 108 per minute or greater. All patients meeting the shock criteria were included in the shock cohort, because a low score may be attributable to poor cerebral perfusion secondary to shock and may not be indicative of severe TBI, and GCS score thus becomes much less reliable as a predictor of TBI in this setting.12 Furthermore, a previous trial of hypertonic resuscitation for patients with out-of-hospital GCS score of 8 or less and systolic blood pressure less than 100 mm Hg had closed for futility, so the current study focused on the effect of these resuscitation strategies for patients with TBI but without evidence of hypovolemic shock.9
Exclusion criteria included known or suspected pregnancy, age younger than 15 years, out-of-hospital cardiopulmonary resuscitation, administration of more than 2000 mL of crystalloid or any amount of colloid or blood products prior to enrollment, severe hypothermia (<28°C), drowning, asphyxia due to hanging, burns on more than 20% of total body surface area, isolated penetrating head injury, inability to obtain intravenous access, more than 4 hours between receipt of dispatch call to study intervention, prisoner status, and interfacility transfer.
Intervention
Out-of-hospital personnel were trained to administer unlabeled study fluid as the initial resuscitation fluid once intravenous access was established. In the event that a participating out-of-hospital crew arrived after crystalloid had already been initiated (eg, by ground service before aero-medical crew arrival), they were allowed to administer study fluid as long as the patient still met inclusion criteria and had received less than 2 liters of crystalloid and had not received any mannitol, colloids, or blood products. Once study fluid had been administered, additional fluids could be given as guided by local emergency medical services protocols. Subsequent in-hospital care was not prescribed, with the exception of protocol-specified monitoring of serum sodium levels during the first 24 hours. Investigators encouraged the implementation of established guidelines for care of critically ill patients with trauma.13
Outcome Measures
The primary outcome was 6-month neurologic status based on the Extended Glasgow Outcome Score (GOSE).14 Additional assessment of neurologic outcome included the GOSE at discharge and 1 month following discharge, and the Disability Rating Score (DRS) at discharge, 1 month following discharge, and 6 months following injury.15 The GOSE and DRS following discharge were determined by the use of a structured telephone survey.16 If the patient was unable to respond to the survey, information was gathered from a family member or caregiver. Previous reports have validated the use of information obtained from caregivers to assess both the GOSE and DRS.14,16,17
Other secondary outcomes included 28-day survival, survival to hospital discharge, ICP, interventions required to manage intracranial hypertension, fluid and blood requirements in the first 24 hours, physiologic parameters of organ dysfunction, 28-day acute respiratory distress syndrome–free survival, Multiple Organ Dysfunction Score,18 and nosocomial infections.19,20 Diagnosis of multiple organ dysfunction was subject to patients having the required physiologic measurements available during their intensive care unit (ICU) stay. Measures of resource utilization included ventilator-free days in the first 28 days, days alive outside the ICU, and days alive outside the hospital within 28 days. TBI severity was assessed using the Abbreviated Injury Score for the head (head AIS) and the Marshall Score to grade the head computed tomography findings.21,22
Randomization and Blinding
All study fluids were purchased from Biophausia Inc (Stockholm, Sweden). Study fluids were provided in identical intravenous bags and shipped to a single distribution center, where they were labeled with a randomly generated numeric code. The randomization scheme was 1:1:1.4 for hypertonic saline, hypertonic saline/dextran, and normal saline, respectively. This ratio was chosen because it can be shown that this is technically the most efficient ratio for this setting.23 Patients were individually randomized by administration of a blinded bag of study fluid.
All out-of-hospital personnel, clinicians, investigators, and patients remained blinded to the treatment assignment until end of study. Because of a labeling issue at the onset of the study, the randomization scheme was initially biased toward enrolling more patients into the normal saline group. After the issue was resolved, randomization continued according to protocol, with final distribution among the groups approaching the planned ratios.
Sample Size and Power Calculations
To assess neurologic outcome, we dichotomized the GOSE into good outcome (moderate disability or good recovery [GOSE >4]) vs poor outcome (severe disability, vegetative state, or death [GOSE ≤4]). A 15% relative reduction in the prevalence of poor outcome was considered clinically relevant. Review of the literature suggested that 40% to 57% of this population would have a poor outcome.24,25
A 49% incidence of poor outcome was estimated, and hypertonic fluids were assumed to offer a 15% relative reduction (absolute reduction, 7.5%) in the risk of poor outcome. Based on a previous trial that used a GCS score of 8 or less as an out-of-hospital enrollment criterion, we anticipated that approximately 10% of the patients enrolled in the TBI cohort would have a less severe injury and have other reasons for altered mental status, such as alcohol or drug intoxication.26 These patients would be unlikely to meet criteria for poor outcome, regardless of treatment. Therefore, we estimated a sample size of 2122 patients to provide an overall power of 80% (1-sided study-wide α=.025, 62.6% power for each of the 2 comparisons) for an attenuated absolute reduction of 6.75% (based on the 10% contamination with truly uninjured patients) for each individual agent vs control, accounting for the primary analysis and 2 interim analyses.
Data Analysis
The primary analysis was designed as modified intent-to-treat, with all patients who had fluid connected to the intravenous tubing included regardless of how much fluid was administered. Per the a priori trial design, patients for whom the fluid bag was opened but not connected to the intravenous line were not included in the primary analysis; thus, we were not permitted to collect hospital and outcome data on such patients because they were not considered enrolled in the trial. Tests for differences in proportions were used for the primary analysis.
Initial analyses of the data indicated the absence of 6-month neurologic outcome data for 15% of the study cohort. Therefore, in addition to the completer analysis, we performed an analysis using multiple hot deck imputations (20 imputations) to estimate the 6-month neurologic outcome. For these imputations we used data from patients who were discharged alive based on 1-month GOSE data or discharge GOSE (if 1-month data were not available), length of hospital stay, and treatment group.27 For 28-day survival, patients with missing 28-day vital status who were known to be discharged alive prior to 28 days were assumed to be alive at day 28.
Significance was defined as P<.05 based on 2-sided tests. Differences in means or proportions with 95% confidence intervals are also presented. Medians with interquartile ranges (IQRs) are reported for skewed variables. Analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) and S-plus version 7.0 (Tibco Spotfire, Somerville, Massachusetts). A priori secondary analyses included patients with an Abbreviated Injury Score for the head (head AIS) of 4 or greater and 2 or greater, those with documented intracranial hemorrhage, and those requiring emergent craniotomy. Secondary outcomes were assessed using t tests or χ2 analyses as appropriate.
Trial Monitoring
Trial monitoring was conducted using a group sequential stopping rule for each comparison of hypertonic saline/dextran vs normal saline and hypertonic saline vs normal saline, based on a level 0.0125 one-sided group sequential test with O’Brien-Fleming boundary relationships for efficacy and a non-binding futility boundary that corresponds to a boundary in the Wang and Tsiatis28 power family of boundary shape functions, as implemented in the unified family of Kittelson and Emerson29 with boundary shape parameter P=.80 and β=.9875. In making a decision to terminate the clinical trial, the data and safety monitoring board was also presented with estimates of the 95% confidence intervals for treatment effects after adjustment for the sequential stopping rule, the Bayesian predictive power of eventual statistical significance based on noninformative (flat) prior distributions, and conditional power estimates defined for a spectrum of hypothesized treatment effects.
Regulatory Oversight
The study was conducted under the US regulations for Exception From Informed Consent for Emergency Research (21 CFR 50.24) and the Canadian Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. The protocol was reviewed and approved by the US Food and Drug Administration and Health Canada. The protocol was also approved by all institutional review boards (United States) and research ethics boards (Canada) in the communities in which the research was conducted. Consent was obtained for continuation in the trial after hospital arrival. Details of the community consultation and public disclosure processes have been published elsewhere.30,31
RESULTS
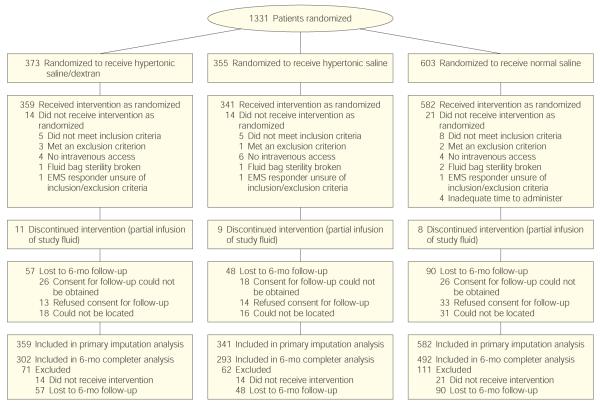
Between May 2006 and May 2009, 1331 patients were randomized (Figure 1). For 49 of these, the study fluid package was opened but fluid was not administered. Reasons included that out-of-hospital personnel recognized that the patient did not meet inclusion criteria or met 1 of the exclusion criteria; intravenous access could not be obtained or was lost prior to fluid administration; the sterility of the bag had been broken; or out-of-hospital personnel were unsure of inclusion/exclusion criteria and elected not to administer the fluid.
Figure 1.
Trial Enrollment
Imputation analysis for 6-month neurologic outcome included all patients who received the intervention. Final completer analysis outcome data included all patients who received the intervention, defined as having the fluid connected to the intravenous line regardless of how much fluid was administered, and who completed 6-month follow-up. EMS indicates emergency medical system.
Complete 6-month neurologic outcome data were available for 1087 of 1282 treated patients (85%). The primary reasons for loss of follow-up included refusal of the patient to consent to contact after discharge, inability to obtain consent because of rapid discharge of minimally injured patients or lack of legal next of kin for severely disabled patients, or inability to locate the patient after discharge. Neurologic outcome data were available at either discharge or 1 month for 1217 of 1282 patients (95%). Because of the nature of this trial we were unable to track patients screened but not enrolled, and adequate epidemiologic data to estimate the potentially eligible population were not available.
At a planned data and safety monitoring board review for the second formal interim analysis (April 27, 2009), data from 1073 participants were reviewed and the futility boundary was crossed, with a boundary crude difference of 0.016 in proportion of good outcome for each of the 2 comparisons of treatment to normal saline. The crude differences for the group comparisons ranged from −0.017 to −0.041, depending on the assumptions made regarding missing values. As a result and independent of how missing values were handled, the futility boundary was crossed for each of the 2 group comparisons (hypertonic saline/dextran vs normal saline and hypertonic saline vs normal saline). Predictive and conditional power comparing each of the treatment groups with the normal saline group were small and ranged from <0.0001% to <3%, depending on the assumptions made regarding the hypothesized effect (noninformative flat Bayesian prior for the predictive power and maximum likelihood estimate or initially hypothesized effect for the conditional power).
There were no significant differences in baseline characteristics, injury severity scores, and out-of-hospital care provided between treatment groups (Table 1). Protocol violations did not differ between the groups. There were no differences in admission vital signs or laboratory studies with the exception of the expected increase in serum sodium level and a slightly lower hemoglobin level in the hypertonic fluid groups (Table 2). As expected, serum sodium level was elevated beyond 12 hours in 36.5% of the patients receiving hypertonic saline compared with 13.4% of those receiving normal saline.
Table 1.
Demographic, Injury Severity, and Out-of-Hospital Care Characteristicsa
| Characteristic | Hypertonic Saline/Dextran (n = 359) |
Hypertonic Saline (n = 341) |
Normal Saline (n = 582) |
|---|---|---|---|
| Age, mean (SD), y | 38.5 (18.6) | 38.6 (17.3) | 39.5 (19.2) |
|
| |||
| Male sex, No. (%) | 273 (76.3) | 277 (81.2) | 426 (73.3) |
|
| |||
| Blunt trauma, No. (%) | 352 (98.6) | 334 (97.9) | 575 (99.0) |
|
| |||
| Out-of-hospital GCS, mean (SD) | 5.0 (2.0) | 4.9 (2.3) | 5.0 (2.1) |
|
| |||
| Median (IQR) | 5.0 (3.0-7.0) | 4.0 (3.0-7.0) | 5.0 (3.0-7.0) |
|
| |||
| RTS, mean (SD) | 4.9 (1.1) | 4.8 (1.2) | 5.1 (1.2) |
|
| |||
| TRISS probability outcome, mean (SD) | 0.65 (0.30) | 0.66 (0.28) | 0.67 (0.30) |
|
| |||
| Median (IQR) | 0.74 (0.41-0.91) | 0.72 (0.42-0.90) | 0.77 (0.43-0.93) |
|
| |||
| ISS, mean (SD) | 26.9 (15.9) | 26.2 (15.3) | 26.1 (15.6) |
|
| |||
| Median (IQR) | 26.0 (17.0-37.0) | 25.0 (17.0-35.0) | 26.0 (14.0-35.0) |
|
| |||
| Head AIS, mean (SD) | 3.3 (1.9) | 3.3 (1.8) | 3.3 (1.8) |
|
| |||
| Score, No. (%) | |||
| 1 | 3 (0.8) | 2 (0.6) | 1 (0.2) |
|
| |||
| 2 | 30 (8.5) | 31 (9.3) | 55 (9.7) |
|
| |||
| 3 | 44 (12.5) | 45 (13.5) | 74 (13.1) |
|
| |||
| 4 | 81 (22.9) | 67 (20.1) | 129 (22.8) |
|
| |||
| 5 | 125 (35.4) | 126 (37.8) | 202 (35.7) |
|
| |||
| 6 | 3 (0.8) | 1 (0.3) | 1 (0.2) |
|
| |||
| 9 (unknown) | 6 (1.7) | 6 (1.8) | 11 (1.9) |
|
| |||
| Marshall Score, first head CT, No. (%) | |||
| Diffuse injury I | 102 (29.2) | 98 (30.2) | 175 (31.2) |
|
| |||
| Diffuse injury II | 128 (36.7) | 122 (37.5) | 184 (32.9) |
|
| |||
| Diffuse injury III | 44 (12.6) | 40 (12.3) | 67 (12.0) |
|
| |||
| Diffuse injury IV | 13 (3.7) | 16 (4.9) | 22 (3.9) |
|
| |||
| Mass lesion | 59 (16.9) | 43 (13.2) | 105 (18.8) |
|
| |||
| Out-of-hospital advanced airway, No. (%) | 224 (62.6) | 212 (62.2) | 338 (58.2) |
|
| |||
| Time from dispatch call to fluid administration, mean (SD), min |
33.7 (18.5) | 34.1 (25.2) | 35.7 (24.1) |
|
| |||
| Median (IQR) | 29.8 (20.9-42.0) | 28.0 (20.0-40.0) | 29.8 (20.3-45.0) |
|
| |||
| Total out-of-hospital time, mean (SD), min | 54.9 (23.8) | 56.0 (30.4) | 57.3 (30.3) |
|
| |||
| Median (IQR) | 51.6 (38.0-66.0) | 47.9 (37.6-65.7) | 51.1 (38.2-68.0) |
|
| |||
| Out-of-hospital fluids, mean (SD), L | 0.88 (0.71) | 0.85 (0.65) | 0.82 (0.63) |
|
| |||
| Median (IQR) | 0.70 (0.35-1.25) | 0.65 (0.35-1.25) | 0.65 (0.35-1.15) |
|
| |||
| Air transport, No. (%) | 150 (41.9) | 139 (40.8) | 217 (37.4) |
Abbreviations: CT, computed tomography; GCS, Glasgow Coma Score; head AIS, Abbreviated Injury Score for the head; IQR, interquartile range; ISS, Injury Severity Score; RTS, Revised Trauma Score, TRISS, probability of survival based on ISS and RTS.
Range for GCS, 3 through 15; for TRISS, 0 through 1; for ISS, 0 through 75; for head AIS, 0 through 6.
Table 2.
Admission Physiology, Neurologic Monitoring, and Interventions
| Mean (SD) | Difference, % (95% CI) | |||||
|---|---|---|---|---|---|---|
| Hypertonic Saline/Dextran (n = 359) |
Hypertonic Saline (n = 341) |
Normal Saline (n = 582) |
P Valuea |
Hypertonic Saline/Dextran vs Normal Saline |
Hypertonic Saline vs Normal Saline |
|
| Admission physiology | ||||||
| Systolic blood pressure, mm Hg | 141.2 (33.1) | 136.9 (33.5) | 139.1 (33.1) | .23 | 2.1 (−2.2 to 6.5) | −2.2 (−6.6 to 2.3) |
|
| ||||||
| GCS score | 5.2 (3.6) | 5.5 (3.8) | 5.4 (3.7) | .64 | −0.2 (−0.7 to 0.3) | 0.1 (−0.4 to 0.6) |
|
| ||||||
| Median (IQR) | 3.0 (3.0-6.0) | 3.0 (3.0-7.0) | 3.0 (3.0-7.0) | |||
|
| ||||||
| Hemoglobin, g/dL | 12.0 (2.2) | 12.5 (2.2) | 13.1 (2.3) | <.001 | −1.0 (−1.3 to −0.7) | −0.6 (−0.9 to −0.3) |
|
| ||||||
| Serum sodium, mEq/L | 146.1 (5.1) | 147.1 (5.1) | 139.1 (3.6) | <.001 | 7.0 (6.4 to 7.6) | 8.1 (7.4 to 8.7) |
|
| ||||||
| Serum sodium >145 mEq/L, No. (%) | ||||||
| 0-4 h | 206 (59.9) | 233 (70.4) | 27 (4.7) | <.001 | 55.1 (49.7 to 60.6) | 65.6 (60.4 to 70.9) |
|
| ||||||
| 4-12 h | 122 (43.0) | 133 (48.0) | 49 (10.6) | <.001 | 32.4 (25.9 to 38.8) | 37.4 (30.9 to 43.9) |
|
| ||||||
| 12-24 h | 93 (36.5) | 96 (36.6) | 58 (13.4) | <.001 | 23.0 (16.3 to 29.8) | 23.2 (16.6 to 29.9) |
|
| ||||||
| Neurologic monitoring | ||||||
| ICP monitoring | ||||||
| Monitored, No. (%) | 105 (29.2) | 93 (27.3) | 155 (26.6) | .68 | 2.6 (−3.3 to 8.5) | 0.6 (−5.3 to 6.6) |
|
| ||||||
| Initial ICP, mm Hg | 17.9 (17.0) | 15.1 (13.3) | 18.6 (14.5) | .22 | −0.7 (−5 to 3.5) | −3.5 (−7.3 to 0.2) |
|
| ||||||
| Highest ICP in the first 12 h, mm Hg |
25.5 (19.9) | 23.6 (18.4) | 25.8 (17.6) | .64 | −0.3 (−5.1 to 4.5) | −2.2 (−6.9 to 2.5) |
|
| ||||||
| Hours with ICP >25 mm Hg in the first 12 h |
1.2 (2.8) | 1 (2.9) | 1 (2.6) | .89 | 0.2 (−0.5 to 0.8) | 0 (−0.7 to 0.7) |
|
| ||||||
| CPP monitoring | ||||||
| Initial CPP, mm Hg | 71.5 (22.0) | 76.3 (23.2) | 72.5 (20.0) | .31 | −1.0 (−6.7 to 4.8) | 3.9 (−2.5 to 10.2) |
|
| ||||||
| Hours with CPP <60 mm Hg in first 12 h |
1.9 (3.4) | 1.5 (2.9) | 1.6 (2.9) | .57 | 0.3 (−0.5 to 1.1) | −0.1 (−0.9 to 0.6) |
|
| ||||||
| Interventions for intracranial hypertension | ||||||
| Total mannitol in first 12 h, g/kg | 6.7 (26.1) | 5.6 (21.6) | 11.2 (28.1) | .19 | −4.6 (−11.3 to 2.2) | −5.7 (−12.0 to 0.7) |
|
| ||||||
| Mannitol in first 5 d, No. (%) | 31 (9.1) | 31 (9.6) | 69 (12.6) | .19 | −3.5 (−7.7 to 0.6) | −3.0 (−7.3 to 1.3) |
|
| ||||||
| Additional hypertonic fluids in first 5 d, No. (%) |
64 (18.8) | 62 (19.3) | 128 (23.4) | .17 | −4.6 (−10.1 to 0.8) | −4.2 (−9.8 to 1.4) |
|
| ||||||
| Hyperventilation, No. (%) | ||||||
| First 24 h | 4 (1.1) | 5 (1.5) | 14 (2.4) | NAa | −1.3a | −0.9a |
|
| ||||||
| First 5 d | 8 (2.2) | 5 (1.5) | 15 (2.6) | .54 | −0.3a | −1.1a |
|
| ||||||
| Ventriculostomy, No. (%) | ||||||
| First 24 h | 40 (11.1) | 40 (11.7) | 68 (11.7) | .96 | −0.5 (−4.7 to 3.6) | −0.0 (−4.3 to 4.3) |
|
| ||||||
| First 5 d | 42 (11.7) | 46 (13.5) | 72 (12.4) | .77 | −0.7 (−4.9 to 3.6) | 1.1 (−3.4 to 5.6) |
|
| ||||||
| Craniotomy, No. (%) | ||||||
| First 24 h | 37 (10.3) | 41 (12) | 76 (13.1) | .45 | −2.8 (−6.9 to 1.4) | −1.0 (−5.4 to 3.4) |
|
| ||||||
| First 5 d | 46 (12.8) | 45 (13.2) | 76 (13.1) | .99 | −0.2 (−4.7 to 4.2) | 0.1 (−4.4 to 4.7) |
Abbreviations: CI, confidence interval; CPP, cerebral perfusion pressure; GCS, Glasgow Coma Score; ICP, intracranial pressure; IQR, interquartile range; NA, not available.
For differences in means/proportions across all 3 treatment groups.
Cells too small for valid interval estimation using normal approximation or forχ2 P value.
Intracranial pressure monitors were placed in 28% of the study population. The median time to ICP monitor placement was 4.7 (IQR, 2.8-8.5) hours in the hypertonic saline/dextran group, 4.8 (IQR, 3.0-7.8) hours in the hypertonic saline group, and 4.7 (IQR, 2.7-7.8) hours in the normal saline group. As shown in Table 2, there were no significant differences between the study groups for initial ICP, subsequent episodes of increased ICP, or decreased cerebral perfusion pressure over the first 12 hours. There also was no statistically significant difference in the interventions for intracranial hypertension including use of mannitol, additional hypertonic saline, and rates of hyperventilation, ventriculostomy, or craniotomy (Table 2).
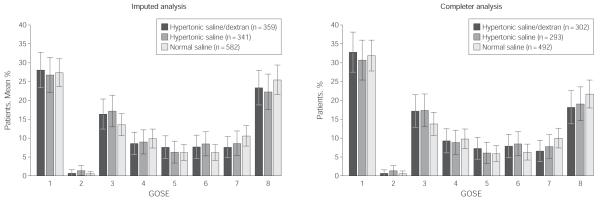
The primary outcome was 6-month neurologic function based on the GOSE. As noted, this outcome measure was obtained for 85% of the study population. To address the possibility that data might not be missing at random, we performed multiple hot deck imputations to account for missing neurologic outcome data, based on patients alive at discharge. Thus, the results for 6-month GOSE are presented as both the completer and imputation analysis, with the multiple imputation analysis results considered the primary result. Figure 2 illustrates the distribution of GOSE by category. When dichotomized to assess GOSE of 4 or less (severe disability, vegetative state, or death), there were no significant differences between the groups based on either the complete cases or imputed analysis (Table 3). There was also no difference in the DRS between treatment groups 6 months after injury.
Figure 2.
Six-Month Extended Glasgow Outcome Score by Treatment Group—Imputed and Completer Analyses
The Extended Glasgow Outcome Scale (GOSE) is an 8-point scale (1=dead, 2=vegetative, 3=lower severe disability, 4=upper severe disability, 5=lower moderate disability, 6=upper moderate disability, 7=lower good recovery, 8=upper good recovery).14 Imputed analysis represents the results for the entire cohort who received the intervention, using multiple hot deck imputations (20 imputations) for missing 6-month GOSE data. Completer analysis represents data from all cases with complete 6-month outcome data, including those who discontinued intervention but received partial infusion of study fluid.
Table 3.
Outcome Measures and Adverse Events
| No. (%) | Difference, % (95% CI) | |||||
|---|---|---|---|---|---|---|
| Hypertonic Saline/Dextran (n = 359) |
Hypertonic Saline (n = 341) |
Normal Saline (n = 582) |
P Valuea |
Hypertonic Saline/Dextran vs Normal Saline |
Hypertonic Saline vs Normal Saline |
|
| 6-mo GOSE ≤4 | ||||||
| Completer analysis | 181 (59.9) | 171 (58.4) | 276 (56.1) | .55 | 3.8 (−3.2 to 10.9) | 2.3 (−4.9 to 9.4) |
|
| ||||||
| Imputed analysis | 192.9 (53.7) | 185.4 (54.3) | 299.8 (51.5) | .67 | 2.2 (−4.5 to 9.0) | 2.9 (−4.0 to 9.7) |
|
| ||||||
| Head AIS | ||||||
| ≥4 | 146.1 (70.2) | 128.0 (66.3) | 219 (66.1) | .59 | 4.1 (−4.1 to 12.3) | 0.1 (−8.5 to 8.7) |
|
| ||||||
| ≥2 | 166.7 (59.3) | 150.6 (56.2) | 253.2 (55.3) | .57 | 4.0 (−3.4 to 11.5) | 0.9 (−6.8 to 8.5) |
|
| ||||||
| DRS categories of disability | ||||||
| 0-1 (none/mild) | 124.8 (34.8) | 128.0 (37.5) | 232.0 (39.9) | .84 | ||
|
|
||||||
| 2-6 (partial/moderate) | 98.3 (27.4) | 89.4 (26.2) | 143.2 (24.6) | |||
|
|
||||||
| 7-21 (moderately severe/severe/ extremely severe) |
33.0 (9.2) | 26.6 (7.8) | 43.3 (7.4) | |||
|
|
||||||
| 22-30 (vegetative/extreme vegetative/ dead) |
102.9 (28.7) | 97.0 (28.4) | 163.4 (28.1) | |||
|
| ||||||
| 28-d survival | 263 (74.3) | 255 (75.7) | 432 (75.1) | .88 | −0.0 (−5.8 to 5.8) | 1.4 (−4.4 to 7.2) |
|
| ||||||
| Survival at hospital discharge | 265 (74.4) | 258 (75.9) | 427 (74.3) | .85 | 0.2 (−5.6 to 5.9) | 1.6 (−4.2 to 7.4) |
|
| ||||||
| ARDS-free survival to day 28 | 247 (69.2) | 242 (71.2) | 404 (70.4) | .84 | −1.2 (−7.3 to 4.9) | 0.8 (−5.3 to 6.9) |
|
| ||||||
| Worst MODS, mean (SD) | 9.0 (9.3) | 8.6 (9.3) | 8.6 (9.5) | .78 | 0.4 (−0.9 to 1.6) | −0.1 (−1.3 to 1.2) |
|
| ||||||
| Median (IQR)b | 6.0 (1.0-24.0) | 5.0 (0.0-13) | 5.0 (0-24.0) | |||
|
| ||||||
| Ventilator-free days, mean (SD) | 16.8 (11.6) | 17.5 (11.5) | 17.4 (11.7) | .67 | −0.6 (−2.2 to 0.9) | 0 (−1.5 to 1.6) |
|
| ||||||
| Median (IQR) | 22 (0-27) | 22.5 (3-27) | 23 (0-27) | |||
|
| ||||||
| Days alive out of ICU to day 28, mean (SD) | 15.1 (11.4) | 15.8 (11.4) | 15.6 (11.7) | .72 | −0.5 (−2.0 to 1.0) | 0.2 (−1.4 to 1.7) |
|
| ||||||
| Median (IQR) | 18.5 (0-26) | 18 (1-27) | 19 (0-27) | |||
|
| ||||||
| Days alive out of hospital to day 28, mean (SD) | 9.8 (10.9) | 10 (11.0) | 10.7 (11.2) | .42 | −0.9 (−2.4 to 0.5) | −0.7 (−2.2 to 0.8) |
|
| ||||||
| Median (IQR) | 4.5 (0-22) | 4.0 (0-22) | 6.0 (0-23) | |||
|
| ||||||
| ≥1 Nosocomial infectionsc | 102 (31.9) | 101 (32.3) | 136 (25.7) | .06 | 6.2 (−0.1 to 12.5) | 6.6 (0.2 to 13.0) |
|
| ||||||
| Pneumonia | 72 (22.5) | 71 (22.7) | 100 (18.9) | .30 | 3.6 (−2.0 to 9.3) | 3.8 (−1.9 to 9.5) |
|
| ||||||
| Bloodstream infection | 23 (7.2) | 21 (6.7) | 19 (3.6) | .04 | 3.6 (0.4 to 6.8) | 3.1 (−0.1 to 6.3) |
|
| ||||||
| Urinary tract infection | 30 (9.4) | 32 (10.2) | 32 (6.0) | .06 | 3.3 (−0.4 to 7.1) | 4.2 (0.3 to 8.1) |
|
| ||||||
| Wound infection | 8 (2.5) | 8 (2.6) | 16 (3.0) | .88 | −0.5d | −0.5d |
|
| ||||||
| Total fluids in first 24 h, mean (SD), L | 6.7 (5.5) | 6.7 (5.5) | 6.9 (5.7) | .68 | −0.3 (−1.0 to 0.5) | −0.3 (−1.0 to 0.5) |
|
| ||||||
| Median (IQR) | 5.2 (3.0-8.6) | 5.2 (3.1-8.5) | 5.3 (3.4-8.6) | |||
|
| ||||||
| PRBC in first 24 h, mean (SD), U | 1.4 (3.6) | 1.2 (3.4) | 1.5 (4.3) | .43 | −0.1 (−0.6 to 0.4) | −0.3 (−0.8 to 0.2) |
|
| ||||||
| Median (IQR) | 0 (0-1) | 0 (0-0) | 0 (0-1) | |||
|
| ||||||
| Hypernatremia (sodium >160 mEq/L) requiring interventionc |
10 (2.9) | 14 (4.2) | 13 (2.3) | .25 | 0.6 (−1.5 to 2.7) | 1.9 (−0.5 to 4.4) |
|
| ||||||
| Increased intracranial hemorrhage on serial head CT |
57 (16.3) | 60 (18.5) | 122 (21.8) | .11 | −5.5 (−10.6 to −0.3) | −3.3 (−8.8 to 2.1) |
|
| ||||||
| Seizures in first 24 h | 4 (1.1) | 3 (0.9) | 12 (2.1) | NAd | −0.9d | −1.2d |
|
| ||||||
| Discharge disposition | ||||||
| Death | 89 (25.3) | 81 (24.1) | 147 (25.6) | .88 | −0.3 (−6.1 to 5.5) | −1.5 (−7.3 to 4.3) |
|
| ||||||
| Home | 138 (39.2) | 134 (39.9) | 253 (44.1) | .26 | −4.9 (−11.4 to 1.6) | −4.2 (−10.8 to 2.4) |
|
| ||||||
| Inpatient rehabilitation | 84 (23.9) | 90 (26.8) | 122 (21.3) | .16 | 2.6 (−3.0 to 8.2) | 5.5 (−0.3 to 11.3) |
|
| ||||||
| Skilled nursing facility | 37 (10.5) | 28 (8.3) | 40 (7.0) | .17 | 3.5 (−0.3 to 7.4) | 1.4 (−2.3 to 5.0) |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CT, computed tomography; DRS, Disability Rating Score; GOSE, Extended Glasgow Outcome Score; head AIS, Abbreviated Injury Score for the head; ICU, intensive care unit; MODS, Multiple Organ Dysfunction Score; NA, not available; PRBC, packed red blood cells.
For differences in means/proportions across all 3 treatment groups.
Calculated as the sum of the worst component scores, unmeasured components estimated as 0.
Percentages based on population at risk.
Cells too small for valid interval estimation using normal approximation or for χ2 P value.
There was no difference in 28-day survival, development of organ failure, or duration of ICU and hospital stay. Interestingly, there appeared to be a higher rate of nosocomial infection in the hypertonic fluid groups, which was primarily related to an increased rate of bloodstream and urinary tract infection. There were no statistically significant differences in the rate of adverse events between the treatment groups. Importantly, we observed no increase in progression of intracranial hemorrhage in the hypertonic fluid groups.
As a predefined subgroup analysis we also evaluated the GOSE between the treatment groups for patients with evidence of severe anatomical TBI (head AIS ≥4) and for those with head AIS of 2 or greater to exclude those with minor or no TBI. As noted in Table 3, restricting the analysis to these subgroups revealed no statistically significant differences among the treatment groups with regard to the proportion of patients with a GOSE of 4 or less.
COMMENT
To our knowledge, this is the largest randomized clinical trial of hypertonic resuscitation following TBI. We were unable to demonstrate any improvement in 6-month neurologic outcome or survival in this patient population. Previous clinical trials in this patient population have been limited and with mixed results. A meta-analysis of the subset of patients with severe TBI enrolled in out-of-hospital studies conducted prior to 1997 of hypertonic saline/dextran for injured patients with hypovolemic shock (n = 233) suggested an odds ratio for improved survival of 2.12 (P = .048). In 2004, Cooper et al9 reported a randomized controlled trial of hypertonic saline vs normal saline for 229 patients with an out-of-hospital GCS score of 8 or less and systolic blood pressure less than 100 mm Hg. No difference in 6-month GOSE was observed between the treatment groups. Mortality in that study was greater than 50%, owing to the fact that those patients had both hypovolemic shock and evidence of severe TBI.
Despite these findings, hypertonic solutions have been increasingly used in the hospital for management of intracranial hypertension in the setting of TBI. Previous studies have used concentrations ranging from 1.6% to 23.4% saline with documented reduction in ICP and improved cerebral perfusion.32-34 No studies have demonstrated improvement in clinical outcome. Data from randomized controlled trials in support of improved neurologic outcome using mannitol, recommended as the primary osmotic agent for management of increased ICP, have likewise been limited.6,35
Because cerebral edema can occur early after injury, we hypothesized that starting hyperosmolar therapy in the out-of-hospital setting could reduce the subsequent sequelae leading to secondary brain injury. It is possible the lack of effect observed is attributable to the need for a more prolonged course of hyperosmolarity continuing into the early hospital period or the dilutional effects of crystalloid therapy, which did not differ after initial treatment. We did not observe any difference in the initial ICP reading between the treatment groups, but this analysis is limited because only 27.5% of the cohort received an ICP monitor and the timing of ICP monitor placement was variable. In most cases these monitors were placed after admission to the ICU; thus, early effects on ICP may not have been detected, and some patients may have died prior to ICP monitor placement. Furthermore, the clinical decision to begin ICP monitoring was made after randomization. Thus, if the initial resuscitation fluid improved the clinical appearance of the patient, this could have resulted in a decrease in the use of ICP monitors in these groups. We did not observe any difference between the treatment groups in the rate of ICP monitoring (Table 2). The increase in serum sodium level observed in the hypertonic fluid groups was consistent with previous studies.9
Despite the plethora of animal data demonstrating modulation of the inflammatory response by hypertonic fluids, we did not observe any difference in rates of organ failure and in fact saw a slight increase in the rate of nosocomial infection in the hypertonic fluid groups. While previous studies have suggested that hypertonicity preserves T-cell function and thus should protect the host from subsequent infection,36-40 recent work has also shown up-regulation of the A3 receptor on neutrophils following injury that could increase susceptibility to infection with hypertonic therapy.41 Further work in this area is necessary to define these mechanisms of action.
Given that patients were enrolled based on the out-of-hospital GCS score, we anticipated that approximately 10% of these patients would have an altered mental status due to intoxicating substances and not significant TBI. To account for this possibility we increased our proposed sample size and planned a subgroup analysis to evaluate outcome for those patients with anatomical evidence of TBI based on a head AIS score of 2 or greater. Restricting the outcome analysis to this subgroup did not show any difference in 6-month GOSE between the treatment groups. Likewise, restricting the analysis to the subgroup of patients with evidence of severe TBI (head AIS ≥4) also did not demonstrate any difference between the treatment groups. This suggests that the inclusion of a wide spectrum of patients with TBI was not the reason for the failure to identify improvement in outcome.
The strengths of this study include the randomized, blinded, placebo-controlled design; large sample size; early administration of hypertonic fluids; and generalizability across several communities in the United States and Canada. The primary limitation is that TBI management in the hospital was not controlled. Therefore, not all patients with severe TBI received ICP monitoring, and the timing of monitor insertion was variable. Furthermore, some patients received additional hypertonic fluids after hospital arrival and others were treated with mannitol, based on neurosurgeon preference. Lastly, the challenge of obtaining complete 6-month follow-up in a trauma population resulted in a 15% rate of missing data for the primary outcome. These data were not likely missing at random, with less severely disabled patients being more difficult to locate along with the ability of surviving patients to refuse continued follow-up. We accounted for this using multiple hot deck imputations to handle missing variables and only imputed data based on patients discharged alive. Conclusions were unchanged when compared with the completer analysis.
In summary, in this randomized controlled trial, we were unable to demonstrate any improvement in 6-month neurologic outcome or survival for trauma patients with presumed severe TBI (out-of hospital GCS ≤8) without evidence of hypovolemic shock, who received a single bolus of hypertonic fluids compared with normal saline in the out-of-hospital setting. While this does not preclude a benefit from such treatment were it administered differently, at present there appears to be no compelling reason to adopt a practice of hypertonic fluid resuscitation for TBI in the out-of-hospital setting.
Acknowledgments
Financial Disclosures: None reported.
Funding/Support: The Resuscitation Outcome Consortium is supported by a series of cooperative agreements with 10 regional clinical centers and 1 data coordinating center (5U01 HL077863, HL077881, HL077871, HL077872, HL077866, HL077908, HL077867, HL077885, HL077887, HL077873, HL077865) from the National Heart, Lung, and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, US Army Medical Research and Materiel Command, the Canadian Institutes of Health Research (CIHR) Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, and the Heart and Stroke Foundation of Canada.
Role of the Sponsors: The American Heart Association has also cosponsored Resuscitation Outcome Consortium research activities.The National Heart, Lung, and Blood Institute had input into the design of the study and review of the manuscript. It did not influence the decision to publish the manuscript. The sponsors were not involved in the collection, management, analysis, or interpretation of the data.
Footnotes
The Resuscitation Outcome Consortium Investigators are listed in the eAppendix.
Online-Only Material: The eAppendix is available at http://www.jama.com.
Additional Contributions: We thank all of the emergency medical services agencies that participated in this project.
REFERENCES
- 1.Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887. doi: 10.1056/NEJM199906173402406. [DOI] [PubMed] [Google Scholar]
- 2.Graham DI, Ford I, Adams JH, et al. Ischaemic brain damage is still common in fatal non-missile head injury. J Neurol Neurosurg Psychiatry. 1989;52(3):346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sell SL, Avila MA, Yu G, et al. Hypertonic resuscitation improves neuronal and behavioral outcomes after traumatic brain injury plus hemorrhage. Anesthesiology. 2008;108(5):873–881. doi: 10.1097/ALN.0b013e31816c8a15. [DOI] [PubMed] [Google Scholar]
- 4.Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30(4):277–290. doi: 10.1007/s10143-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 5.Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009;65(6):1035–1042. doi: 10.1227/01.NEU.0000359533.16214.04. [DOI] [PubMed] [Google Scholar]
- 6.Bratton SL, Chestnut RM, Ghajar J, et al. Brain Trauma Foundation. American Association of Neurological Surgeons. Congress of Neurological Surgeons. Joint Section on Neurotrauma and Critical Care, AANS/CNS Guidelines for the management of severe traumatic brain injury, II: hyperosmolar therapy. J Neurotrauma. 2007;24(suppl 1):S14–S20. doi: 10.1089/neu.2007.9994. [DOI] [PubMed] [Google Scholar]
- 7.Doyle JA, Davis DP, Hoyt DB. The use of hypertonic saline in the treatment of traumatic brain injury. J Trauma. 2001;50(2):367–383. doi: 10.1097/00005373-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Wade CE, Grady JJ, Kramer GC, Younes RN, Gehlsen K, Holcroft JW. Individual patient cohort analysis of the efficacy of hypertonic saline/dextran in patients with traumatic brain injury and hypotension. J Trauma. 1997;42(5)(suppl):S61–S65. doi: 10.1097/00005373-199705001-00011. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DJ, Myles PS, McDermott FT, et al. HTS Study Investigators Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury. JAMA. 2004;291(11):1350–1357. doi: 10.1001/jama.291.11.1350. [DOI] [PubMed] [Google Scholar]
- 10.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of emergency medical service systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11(4):369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 11.Brasel KJ, Bulger E, Cook AJ, et al. Resuscitation Outcomes Consortium Investigators Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg. 2008;206(2):220–232. doi: 10.1016/j.jamcollsurg.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Davis DP, Vadeboncoeur TF, Ochs M, Poste JC, Vilke GM, Hoyt DB. The association between field Glasgow Coma Scale score and outcome in patients undergoing paramedic rapid sequence intubation. J Emerg Med. 2005;29(4):391–397. doi: 10.1016/j.jemermed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Glue Grant Web site [Accessed September 30, 2009];Inflammation and host response to injury clinical guidelines. 2009 http://www.gluegrant.org/clinical-protocols.htm.
- 14.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport M, Hall KM, Hopkins K, Belleza T, Cope DN. Disability rating scale for severe head trauma. Arch Phys Med Rehabil. 1982;63(3):118–123. [PubMed] [Google Scholar]
- 16.Pettigrew LE, Wilson JT, Teasdale GM. Assessing disability after head injury. J Neurosurg. 1998;89(6):939–943. doi: 10.3171/jns.1998.89.6.0939. [DOI] [PubMed] [Google Scholar]
- 17.Hall K, Cope DN, Rappaport M. Glasgow Outcome Scale and Disability Rating Scale: comparative usefulness in following recovery in traumatic head injury. Arch Phys Med Rehabil. 1985;66(1):35–37. [PubMed] [Google Scholar]
- 18.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple Organ Dysfunction Score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, et al. The Consensus Committee Report of the American-European consensus conference on ARDS. Intensive Care Med. 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 20.Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The International Sepsis Forum Consensus Conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33(7):1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 21.Walder AD, Yeoman PM, Turnbull A. The Abbreviated Injury Scale as a predictor of outcome of severe head injury. Intensive Care Med. 1995;21(7):606–609. doi: 10.1007/BF01700170. [DOI] [PubMed] [Google Scholar]
- 22.Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(9)(suppl 1):S287–S292. [PubMed] [Google Scholar]
- 23.Sheffe H. The Analysis of Variance. Wiley Inc; New York, NY: 1959. [Google Scholar]
- 24.Cooke RS, McNicholl BP, Byrnes DP. Use of the Injury Severity Score in head injury. Injury. 1995;26(6):399–400. doi: 10.1016/0020-1383(95)00064-g. [DOI] [PubMed] [Google Scholar]
- 25.Vos PE, van Voskuilen AC, Beems T, Krabbe PF, Vogels OJ. Evaluation of the traumatic coma data bank computed tomography classification for severe head injury. J Neurotrauma. 2001;18(7):649–655. doi: 10.1089/089771501750357591. [DOI] [PubMed] [Google Scholar]
- 26.Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma. 2003;54(3):444–453. doi: 10.1097/01.TA.0000053396.02126.CD. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley Inc; Hoboken, NJ: 1987. [Google Scholar]
- 28.Wang SK, Tsiatis AA. Approximately optimal one-parameter boundaries for group sequential trials. Biometrics. 1987;43(1):193–199. [PubMed] [Google Scholar]
- 29.Kittelson JM, Emerson SS. A unifying family of group sequential test designs. Biometrics. 1999;55(3):874–882. doi: 10.1111/j.0006-341x.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 30.Tisherman SA, Powell JL, Schmidt TA, et al. Resuscitation Outcomes Consortium Investigators Regulatory challenges for the resuscitation outcomes consortium. Circulation. 2008;118(15):1585–1592. doi: 10.1161/CIRCULATIONAHA.107.764084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulger EM, Schmidt TA, Cook AJ, et al. ROC Investigators The random dialing survey as a tool for community consultation for research involving the emergency medicine exception from informed consent. Ann Emerg Med. 2009;53(3):341–350. doi: 10.1016/j.annemergmed.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn P, Münch E, Vajkoczy P, et al. Hypertonic saline solution for control of elevated intracranial pressure in patients with exhausted response to mannitol and barbiturates. Neurol Res. 1999;21(8):758–764. doi: 10.1080/01616412.1999.11741010. [DOI] [PubMed] [Google Scholar]
- 33.Ware ML, Nemani VM, Meeker M, Lee C, Morabito DJ, Manley GT. Effects of 23.4% sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57(4):727–736. [PubMed] [Google Scholar]
- 34.Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 35.Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2005;(4) doi: 10.1002/14651858.CD001049.pub2. CD001049. [DOI] [PubMed] [Google Scholar]
- 36.Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42(4):190–196. [PubMed] [Google Scholar]
- 37.Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003;278(7):4590–4596. doi: 10.1074/jbc.M207868200. [DOI] [PubMed] [Google Scholar]
- 38.Coimbra R, Hoyt DB, Junger WG, et al. Hypertonic saline resuscitation decreases susceptibility to sepsis after hemorrhagic shock. J Trauma. 1997;42(4):602–607. doi: 10.1097/00005373-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Coimbra R, Junger WG, Hoyt DB, Liu FC, Loomis WH, Evers MF. Hypertonic saline resuscitation restores hemorrhage-induced immunosuppression by decreasing prostaglandin E2 and interleukin-4 production. J Surg Res. 1996;64(2):203–209. doi: 10.1006/jsre.1996.0329. [DOI] [PubMed] [Google Scholar]
- 40.Coimbra R, Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic/hyperoncotic fluids reverse prostaglandin E2 (PGE2)-induced T-cell suppression. Shock. 1995;4(1):45–49. doi: 10.1097/00024382-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Inoue Y, Chen Y, Pauzenberger R, Hirsh MI, Junger WG. Hypertonic saline up-regulates A3 adenosine receptor expression of activated neutrophils and increases acute lung injury after sepsis. Crit Care Med. 2008;36(9):2569–2575. doi: 10.1097/CCM.0b013e3181841a91. [DOI] [PMC free article] [PubMed] [Google Scholar]