Abstract
Introduction
Maximal oxygen uptake (VO2 max) is associated with cardiovascular and metabolic risks but it is difficult to assess in obese children. The objective of this study was to develop an equation to estimate VO2 (mL/kg/min) and to check the % of tests that were maximal according to recommended criteria.
Methods
Stress tests were analyzed of 222 subjects (94 male and 128 female with a BMI above the 85 percentile for age and sex), and repeated 4 months later. Mean age was 9.4 ± 1.1 years and weighed 52.4 ± 13.3 kg. Body fat % (40.5 + 6.2) was determined by DXA (Hologic QDR 4500W).
The protocol on the treadmill started with a warm up at 2.5 and 3 mph with a slope of 0% and 2%. The speed was kept at 3 mph for all the stages and the slope was increased 2% every 2 minutes. Statistical analysis (descriptive, t-test and ANOVAS 2×2×2) was done with SPSS 15.0.
Results
Only 35% of the tests were maximal. The equation calculates was Y = 2.6x + 22.3 (x = protocol stage). Data pre and post treatment were not statistically different
Discussion
Increments in VO2 were consistent despite subject diversity (sex, % body fat, physical fitness, treatment).
Conclusion
To be able to estimate VO2 at the different stages of the test without complex equipment or specialized staff, will facilitate the performance of stress tests on a daily basis.
Introduction
The increase in the rates of obesity in all population groups in the last 20 years the world over has been confirmed by a large quantity of data published in the scientific literature. According to the World Health Organization (WHO), in 1995 there were an estimated 200 million obese adults worldwide and another 18 million children of less than 5 years of age classified as overweight. The number of adults had increased to over 300 million by 2000 and at least 400 million adults were obese in 2005 and the WHO further predicts that by 2015 more than 700 million will be obese (WHO, 2004; WHO, 2006).
In almost all the developed countries, the prevalence has been increasing in a similar way in children and adolescents. At least 20 million children under the age of 5 years were overweight in 2005 (WHO, 2006). These trends are extremely important as excess weight and obesity in childhood are significant predictors of obesity in adulthood, with experts considering that there is an 80% chance of overweight or obese children carrying this problem into adult life (Whitaker and Wright, 1997).
Simultaneously with the appearance of an epidemic increase in obesity including children (Fagot-Capagna, Pettitt et al., 2000; Pinhas-Hamiel, Dolan et al., 1996; Troiano and Flegal, 1998; Straus and Pollack, 2001) there has been an epidemic increase in type-2 diabetes in both children and adults (Mokdad, Serdula et al., 1996; Harrel, Mc Murria et al., 2000) and the WHO (2006) projects that diabetes deaths will increase by more than 50% worldwide in the next ten years.
Although many factors are involved, basically the cause of too much fat being deposited depends on the imbalance between energy consumption and expenditure (Swinburn, Jolly et al., 2006) and although its degree of importance can be debated, physical inactivity is considered to play a major role in the development of obesity (Powell and Blair, 1994) and some authors think that vigorous activity is necessary to provide the mechanical stimulation that leads stem cells to differentiate into bone and muscle instead of fat (Gutin, 2008; Rosen et al., 2006; Rubin et al., 2007).
Maximum oxygen uptake (VO2 max) is generally considered to be the best measure of cardio respiratory fitness (CRF) (Freedson and Goodman, 1993). Moreover, although VO2 max has a major genetic component, it is greatly influenced by the subjects’ activity, thereby serving also to assess activity levels (Siconolfi, Lasater et al., 1985). This has meant that the determination of VO2 max is a routine procedure in research projects to assess the cardiovascular capacity (CVC) of the subjects. Eriksson and Lindgarde (1996) found that Swedish men who developed type-2 diabetes within 6 years of testing had 16% lower VO2 max values than normal and insulin sensitivity has been positively related with physical fitness in adults (Clausen, Borch-Jonen et al., 1996; Endre, Mattiasson et al. 1994; Nyholm, Mengel, et al., 1996) and lower VO2 has been linked with a greater concentration of small, dense LDL and an unfavourable HDL profile in healthy young men (Clausen, Borch-Jonen et al., 1996; Endre, Mattiasson et al., 1994; Nyholm, Mengel, et al., 1996). In the same way lower levels of physical and vascular fitness are associated with cardio-metabolic risk in youth as well as elevated body fatness (Andersen et al., 2006; Gutin et al., 2004; Krekoukia et al., 2007) and because of the strong inverse correlation between CRF and fatness it is possible that deleterious consequences ascribed to adiposity may be partially due to the influence of a lack of CRF (Gutin, 2005).
In spite of its usefulness, to try to carry out determinations of VO2 max in children and especially in overweight or obese children is not an easy task. The common result is that a more or less significant proportion does not fulfil the requisites to be able to consider that they reached VO2 max in their tests (Buono, Roby et al., 1991; Rivera-Brown, Rivera et al., 1992; Armstrong and Welsman, 1994; Rivera-Brown, Rivera et al., 1994; Rivera-Brown, Rivera et al., 1995; Duncan and Howley, 1999; Gutin et al., 2002). Apart from the possible lack of physical fitness and motivation, there is a series of problems which make it difficult to generalize the procedures for testing overweight and obese children. Among them we could cite the need for costly equipment, personnel specialized in the administration of the protocols used and having to collect samples of expired air, which independently of the procedure used, make the task of walking or running on a treadmill or pedalling on a cycle ergometer, uncomfortable (Rivera-Brown, Rivera et al., 1992; Armstrong and Welsman, 1994; Rivera-Brown, Rivera et al. 1994; Rivera-Brown, Rivera et al., 1995; Duncan, Mahon et al., 1996).
In many situations which do not require the precision of a direct VO2 determination recourse is made to procedures for estimating uptake based on the relation that exists between the intensity of effort or work load and oxygen uptake. VO2 consumption estimates can be used to ascertain the energy expenditure, but they must take into account the greater or lesser economy of the work performed (Wasserman, Hansen et al., 1987). These authors indicate how adult obese subjects have a greater oxygen uptake when walking and therefore greater energy expenditure at submaximal intensities. Several authors have also indicated the lower economy of children compared to adults when doing physical activity and consequently the use of adult models to predict energy cost in weight-bearing activities fails to account for the increased energy costs in children (McArdle, Katch et al., 2001; Krahenbulhl and Pangrasi, 1983; Krahenbuhl, Pangrazi, et al., 1989; Ariëns et al., 1998) and especially in obese children (Bar-Or, 1983). There is presently no specific equation to estimate oxygen uptake for obese children, therefore it would be extremely useful to know the actual uptake of these children and provide a specific equation to estimate their oxygen uptake and thus their energy expenditure based on the workload on the treadmill. Due to the relation among oxygen uptake, work load and heart rate, the individual relation between heart rate and work load determined in a progressive stress test will permit the estimation of energy expenditure in these subjects.
The purpose of this analysis was to determine relative VO2 (ml/kg/min) in a group of overweight or obese children at different stages of a treadmill stress test with a modified protocol proposed by Rowland (1993) and the ACSM (2000) to provide a specific equation to estimate relative VO2 in order to obviate its direct measurement in practical evaluations of overweight children. As the energy expenditure for a given workload is relatively constant, relative VO2 at a given stage during the treadmill test will not be affected by physical training. An additional purpose of the study was to check the proportion of tests that could be considered maximal.
Methods
Subjects
A total of 222 children (age range 7.0 to 11.9; mean = 9.4; SD = 1.1) were selected in the 6 cohorts that participated in the PLAY project carried out at the Georgia Prevention Institute (GPI) at the Medical College of Georgia (MCG). Of the subjects 94 were male and 128 female, 93 white and 129 African American, all of whom were recruited through school flyers from elementary schools in the area.
Prior to the tests analyzed below the children and their parents had signed informed assent and consent forms as part of the protocol to participate in the Project. They were chosen if their Body Mass Index (BMI) was above the 85th percentile for their age and gender according to the norms supplied by the CDC (Ogden, Kuczmarski et al., 2002). Children with significant health problems (e.g. orthopaedic limitations, cardiac conditions, etc.) or medication that could affect the results of the study or preclude their safe participation were excluded. The population of obese children was selected because of our interest in amelioration of obesity and the GPI’s background working with this type of subjects.
Some of the children did not have adequate data for the pre-stress test (10 children) and data of 2 additional tests were not accurate enough to be used for the analysis due to equipment malfunctioning, so, the data of 210 pre-tests and 193 (17 dropped out) post-tests repeated four months later were used for the analysis.
The children participating in the Project were randomly assigned to one of three groups: a control group with 0 minutes of exercise, one with 20 minutes and the other with 40 minutes. The program was carried out over 4 months, 5 days a week in the gym of the GPI. During these training periods the children carried out different activities which raised their heart rate during the session to over 150 beats per minute.
Measurements
Weight (in shorts and t-shirt) was measured on an electronic scale and height on a stadiometer. Body composition was determined by DXA with a Hologic QDR 4500W (Hologic Inc. Bedford MA) that determines body composition in three compartments: fat, bone and fat-free soft tissue. Sexual maturation state was assessed by a paediatrician using the Tanner stages and whether the girls had experienced menarche.
The treadmill test consisted of a multi-stage protocol modified from the protocol proposed by Rowland (1993) and the ACSM (2000) for poorly fit children with an additional warm-up period of 2.5 mph (4.02 km/h) and 0% slope for 2 minutes before the warm-up at 3 mph (4.83 km/h) with a 3% slope for 2 minutes proposed in the original protocol. After the warm-up the speed remained at 3 mph but the slope increased by 2% every two minutes until the children decided to stop, or until VO2 max was reached. Sometimes it was stopped for safety reasons (e.g. gait problems). The criteria used to consider the test as maximal were if the child achieved at least 2 of the 3 following conditions: a) a plateau of VO2 over the last two work rates, i.e. less than 0.100 L/min (2 mL/kg/min) increase; b) a plateau of the heart rate over the last work rates, i.e. less than a 5 bpm increase; and c) a respiratory exchange ratio (RER) greater than 1.00. Oxygen uptake was measured with a Sensormedic Metabolic System (Vmax 12 l-a) and heart rate was recorded with a Polar pulsometer model S610i, which registered values during the last 15 seconds of each minute. Rate of Perceived Exertion (RPE) according to Börg’s 20 points scale (1974) was recorded as an indicator of the intensity of effort.
Statistical analyses
The data selected on the participating children were analyzed using SPSS 15.0 with descriptive statistics. For the data analysis oxygen uptake values were grouped for the different stages and 1.9% of the warm-up data, and 0.9%, 1.4%, 0.7%, 1.5% for stages 1–4, which were more than 1.5 of the interquartile distance, were considered outliers and eliminated for the typification of the relative VO2 (mL/kg/min) for the different stages.
Difference in economy of effort between children and adults was assessed using a one-sample ttest comparing values obtained with the values calculated using the formula proposed by the ACSM (2000: 303) to estimate oxygen uptake in adults at the different stages.
The average values for relative VO2 (mL/kg/min) at the different stages before and after the intervention, and absolute VO2 (L/min) were compared using a two-tailed t-test for correlated data in the first case and for independent data in the second one. For the analysis of percentage body fat the values were categorized in two groups using the median as reference (Mdn = 41.4%).
The differences for the uptake values for race, gender and categories of % body fat at each stage in the pre and post tests were compared with a two-tailed three-way ANOVA (2 × 2 × 2). While no changes in relative VO2 were anticipated for a given stage due to intervention, in order to see whether the physical training improved economy of movement, the VO2 values for the groups with different exercise doses (0, 20 and 40 minutes) were analyzed at post-test using a two-tailed one-way ANOVA at each stage. The results obtained made it unnecessary to carry out an “a posteriori” test. All statistical analyses were performed with the level of significance fixed at α = 0.05.
As the ANOVA analysis showed significant differences for % of body fat in stages 4 and 5 and interaction between races in stage 5, a break-down of the relative VO2 values of categories of % body fat at stages 4 to 5 and for race at stage 5 was done a posteriori to elucidate practical differences among them. The goodness of fit of the data of children that reached stages 4 to 7 at the end of the program was tested by a χ2 at an α level equal or below .05.
Results
The physical characteristics of the children are presented in table 1. All the parameter values were similar for African-American and white children of both genders. Table 2 shows maturation stages of the children in the study. Only 5 girls had experienced menarche at the beginning of the program and a total of 7 when the second test was administered at the end of the intervention. The highest stage experienced by boys was 4. Only 4 boys had reached it at the beginning of the program and 8 boys had reached it at the end.
Table 1.
Physical characteristics of the children at baseline
| N of subjects | Age (y) | Weight (kg) | Height (cm) | Body fat % | ||
|---|---|---|---|---|---|---|
| Afro. Amer. | ||||||
| Male | 44 | 9.5±0.9 | 51.5±12.2 | 142.8±7.9 | 40.4±6.2 | |
| Female | 76 | 9.5±1.1 | 52.6±12.6 | 141.1±8.0 | 40.9±6.8 | |
| Total | 120 | 9.5±1.0 | 52.2±12.4 | 141.7±8.0 | 40.7±6.8 | |
| White | ||||||
| Male | 44 | 9.3±1.1 | 52.1±12.4 | 141.5±9.5 | 39.7±4.7 | |
| Female | 46 | 9.4±1.1 | 54.5±16.7 | 142.5±11.5 | 40.3±6.4 | |
| Total | 90 | 9.3±1.1 | 53.4±16.7 | 142.0±10.0 | 40.0±5.6 | |
Total number = 210
Table 2.
Tanner’s maturation stages of the participants at pre and post test.
| Breast | Testes Development | Pubic Hair Development | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Female | Male | |
| Pre–Post | Pre–Post | |||||
| Stage 1 | 74 | 59 | 90 | 78 | 76 - 58 | 82 – 66 |
| Stage 2 | 24 | 25 | 4 | 8 | 32 - 32 | 11 –19 |
| Stage 3 | 25 | 27 | 0 | 2 | 16 – 22 | 1 – 2 |
| Stage 4 | 4 | 7 | 4 – 8 | |||
| Stage 5 | 5 | 2 | ||||
| Menarche | 5 | 7 | ||||
Only 35% (138) of the tests reviewed could be considered maximal according to the standard criteria, and in 190 cases the children (49%) stated that they had stopped the test because of pain in their legs (feet, ankles, thighs, calves, legs). The RPE was clearly invalid according to the criteria proposed by Börg (1974) in 311 of the tests (79%) and in the rest it was often evident that they could be discarded because in spite of being progressive, the values were very low at the end of the test or very high at the beginning or did not bear any relation with the heart rate when multiplied by 10, which suggests that the children had not understood what was intended with this rating.
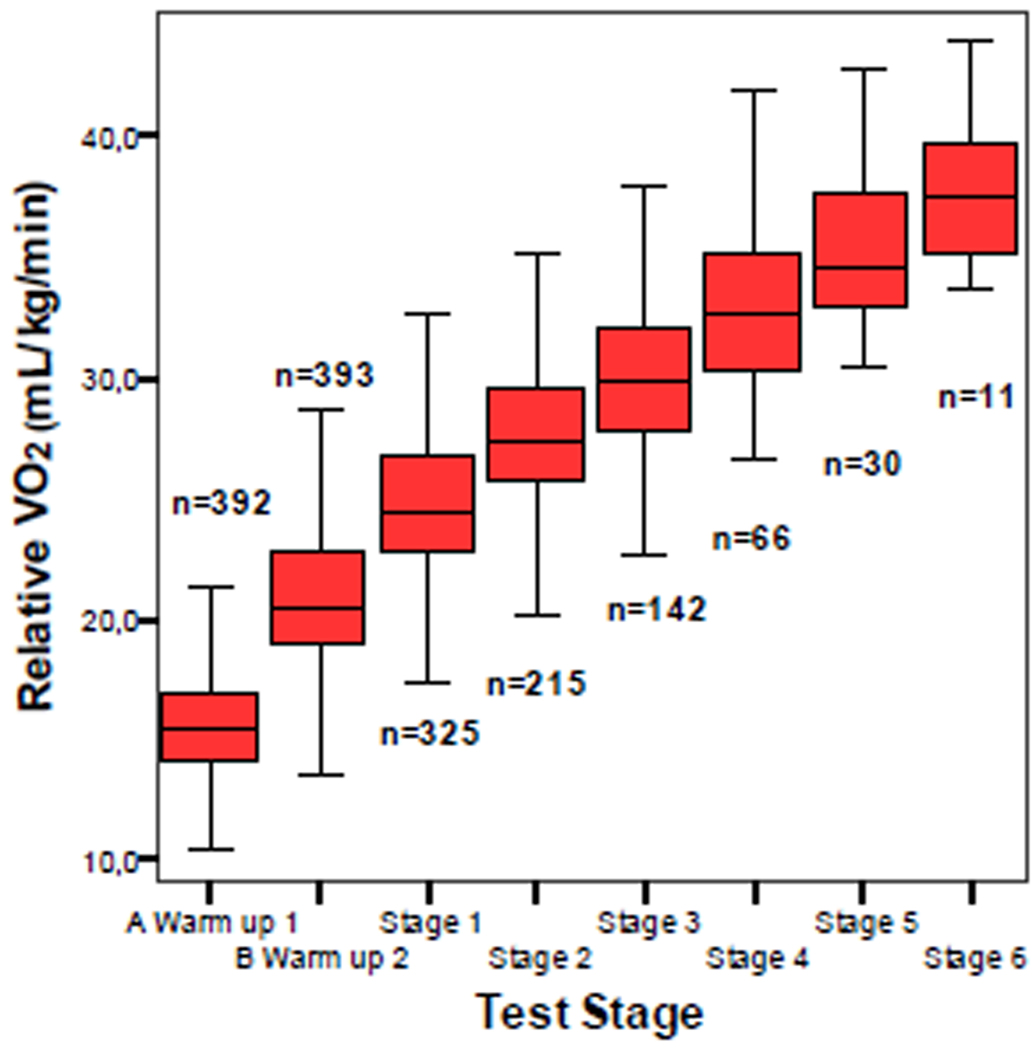
Figure 1 shows the evolution of relative VO2 during the warm-up and the different stages of the test once the outliers had been discarded. It is evident that after the warm-up the increase in oxygen uptake at the different stages was almost linear. Table 3 presents the values of VO2 for the different stages. The equation for the straight line using the mean values was y = 2.6x + 22.3; where x = the order of the stage in the protocol. If the equation was calculated using the median values the results were practically the same, 2.6x + 22.2. So the increment of the slope of the treadmill by 2% implied an average increase of 2.6 mL/kg/min. Table 3 also shows how the values of the mean and the median were practically the same. The differences in all the cases were below 0.5 mL. These data compared to the total data including the outliers were practically the same as well. Differences were below 0.4 mL. Table 3 includes the values of estimated uptake for adults according to the formula proposed by the ACSM (2000: 303). The increase in these values is 2.9 mL/kg/min while that for the children was only 2.6 mL/kg/min. Intercept was very different also. All the values at the different stages were significantly greater than the reference values calculated with the ACSM formula (ps < .001). The range of the values (maximum – minimum) showed a trend to decrease starting with stage 1 as the number of subjects that completed higher stages decreased (Figure 1 and table 3).
Figure 1.
Relative oxygen uptake (consumption) during warm-up and the different stages. The “n” indicates the number of observations used in the analysis.
Table 3.
Descriptive values of oxygen uptake (VO2) and estimated VO2 (VO2 Est) for adults during the warm-up and the different stages of the protocol used and differences.
| Observations | Mean (SD) VO2 (mL/kg/min) |
Median VO2 (mL/kg/min) |
VO2 Est for adults (mL/kg/min) |
Difference vs Adults |
Range of Values |
|
|---|---|---|---|---|---|---|
| Warm-up 1 | 392 | 15.5 ± 2.2 | 15.4 | 6.7 | - | 11.0 |
| Warm-up 2 | 393 | 20.9 ± 2.8 | 20.5 | 12.4 | - | 15.1 |
| Stage 1 | 325 | 24.8 ± 3.0 | 24.5 | 16.7 | 8.1 | 15.4 |
| Stage 2 | 215 | 27.6 ± 2.9 | 27.5 | 19.6 | 8.0 | 17.2 |
| Stage 3 | 142 | 30.1 ± 3.1 | 29.9 | 22.5 | 7.6 | 15.3 |
| Stage 4 | 64 | 32..9 ± 3.2 | 32.7 | 25.4 | 7.5 | 15.3 |
| Stage 5 | 29 | 35.5 ± 3.2 | 34.6 | 28.3 | 7.2 | 12.3 |
| Stage 6 | 12 | 38 ± 3.5 | 37.6 | 31.2 | 6.8 | 10.2 |
(−) Not compared
Prior to testing the different hypotheses, the equality of error variances were checked with Levene’s tests and proved to be insignificant except for the comparison of the absolute values of VO2 at stages 1 and 5 in which cases the tests were done with equal variances not assumed. When comparing the uptake values obtained in the pre- and post-tests the only difference was found in the first warm-up stage, for t (174) = 2.8; p < 0.01. Borderline results were found comparing the values of warm-up 2, t (174) = 1.8; p = 0.07; thus warm-up data were not used in further analyses. No differences were found in relative VO2 between pre and post at any of stages 1–5 (ps >.17), indicating the consistency of the data.
However, as was to be expected when comparing the groups with respect to their greater or lesser % body fat (median split = 41.4 %), the absolute VO2 values were significantly greater in the stages 1–5 of the exercise test given the greater weight due to a higher % body fat (ps < 0,03).
The three-way ANOVA for sex, race and % body fat categories to test differences in total relative oxygen uptake values at each stage of the test only showed interactions between % body fat and race in stage 5 F (1, 23) = 4.85; p = 0.04. Main effects for % body fat were significant at stages 4 and 5, F (1, 58) = 5.07; p = 0.03 and F (1, 23) = 5.67; p = 0.03 respectively. Borderline results were found comparing gender and race in stage 4, F (1, 58) = 2.87; p = 0.095 (Table 4). Table 5 shows an a posteriori break-down of the relative VO2 values of categories of % body fat at stages 4 to 5 and for race at stage 5 to elucidate practical differences among them.
Table 4.
Significant results of the three-way ANOVA for gender × race × % body fat categories in the pre and post tests.
| Source | df | F | Sig. | |
|---|---|---|---|---|
| Stage 4 | % Body Fat Categories | 1 | 5.069 | 0.028* |
| Gender | 1 | 0.382 | 0.539 | |
| Race | 1 | 0.784 | 0.386 | |
| % Body Fat Categories × Gender | 1 | 1.517 | 0.223 | |
| % Body Fat Categories × Race | 1 | 0.324 | 0.572 | |
| Gender × Race | 1 | 2.874 | 0.095 | |
| % Body Fat Categories × Gender × Race | 1 | 1.362 | 0.248 | |
| Error | 58 | |||
| Stage 5 | % Body Fat Categories | 1 | 5.668 | 0.026* |
| Gender | 1 | 1.375 | 0.253 | |
| Race | 1 | 1.109 | 0.303 | |
| % Body Fat Categories × Gender | 1 | 0.195 | 0.663 | |
| % Body Fat Categories × Race | 1 | 4.848 | 0.038* | |
| Gender × Race | 1 | 0.644 | 0.430 | |
| % Body Fat Categories × Gender × Race | 1 | --- | --- | |
| Error | 23 | |||
p < .05 ; ANOVA for stage 6 was not calculated because of the low number of data in cells. (---) Insufficient data.
Table 5.
Break-down of relative VO2 of categories of body fat and race in stages 4 and 5 of the test protocol.
| N | Mean | Std. Deviation | |
|---|---|---|---|
| Fat category > Mdn | |||
| Stage 4 | 24 | 34.3 | 3.1 |
| Stage 5 | 11 | 36.6 | 3.2 |
| Fat category ≤ Mdn | |||
| Stage 4 | 42 | 32.1 | 3.1 |
| Stage 5 | 19 | 34.9 | 3.0 |
| Fat category Total | |||
| Stage 4 | 66 | 32,9 | 3.2 |
| Stage 5 | 30 | 35.5 | 3.2 |
| Race Category | |||
| White Stage 5 | 8 | 36.5 | 4.4 |
| African-American | |||
| Stage 5 | 22 | 35.2 | 2.6 |
| Race Category Total | |||
| Stage 5 | 30 | 35.5 | 3.2 |
When comparing the differences between the different doses of the training groups at post-test with one-way ANOVA, none were found in the stages 1–6 ps > 0.40.
Table 6 shows the significant increment revealed in the percentage of children that completed stages 4 to 7 at the post test after the intervention.
Table 6.
Chi-Square for the percentages of stages 4 to 7 reached by the different treatment groups at the post test compared to the pre test.
| Group | Observed | Expected | χ2 | df |
|---|---|---|---|---|
| Control (0 minutes) | 24.1 | 16.9 | 7.5* | 2 |
| 20 minutes | 14.1 | 9.9 | ||
| 40 minutes | 20.0 | 6.0 |
p < .05
Discussion
The fact that only 35% (138) of the tests reviewed could be considered maximal according to the standard criteria, and in 190 cases the children (49%) stated that they had stopped the test because of pain in their legs (feet, ankles, thighs, calves, legs) demonstrated the necessity of looking for some alternatives when testing obese children. Some alternatives to assess cardiovascular fitness (CVF) have been proposed. Extrapolating the values to a heart rate of 170 bpm o using the heart rate values at a submaximal load have been proposed (Barbeau et al., 2003; Gutin et al., 1997). In this study the mean % body fat of the children was 40.0±5.6 and the utility of using an extrapolation to a value that was far from being reached in many of the children was questionable. Despite the fact that values at a submaximal work load can be useful to show changes in CVF, the use of the values of oxygen uptake and their relations with work load and/or heart rate to calculate energy expenditure would not have been possible. These findings justified the need for a specific equation to estimate VO2 and make possible the computation of energy expenditure.
As expected, in the analysis no differences were found in relative VO2 (mL/kg/min) between pre and post intervention at any of the stages 1 to 5 (ps > 0.17). The data were very consistent. The absolute values (L/min) at these stages were higher for the group with a higher percentage of body fat as they had to move a greater body weight which limited their work capacity. The regression equations calculated for the mean and the median were practically the same due to their minimal differences of intercept (0.1 mL/kg/min) which have no practical importance. Relative values can be estimated from regression equations knowing the stages completed during the stress test.
The characteristics of the group with children of both sexes, with very high percentages of body fat and a very variable and poor fitness level, which has a considerable influence on the speed with which the cardio respiratory system adjusts during the warm-up and the different stages (Whipp, Seard et al. 1970; Simon, Veress et al. 1977) were very heterogeneous and could explain the differences in response during the warm-up. Not to have found statistically significant differences between relative VO2 pre and post test data for stages 1–5 combining all data in the comparison is remarkable and excluded the possibility of changes in the relative VO2 as a consequence of this relatively short period of physical training.
Interaction between race and % body fat was significant only for stage 5 (p = 0.04) that was completed only by 8 white and 22 African-American subjects which made it difficult to draw any conclusions. Main effects were significant for % body fat at stages 4 and 5 (ps = 0.03) and borderline results were found comparing gender and race in stage 4 (p = 0.10) (Table 4). Data were consistent for stages 1 to 3.
The break-down of the values with regard to sex and race indicate that the differences encountered have no practical importance for estimating relative oxygen uptake at stages 4 and 5 (mean difference 0.3 mL/kg/min, range = + 1.4 to − 0.8). See Table 5.
The variability of the results at stages 4 and 5 can be explained in terms of the group’s characteristics with very high levels of body fat and a very variable fitness level which as reported by Wasserman et al (1987) with unfit obese adults, or Bar-Or (1983) with obese children, in some cases would result in a slower increase in uptake (differences at the warm-up), while in others the greater increase could be expected as a consequence of a lesser economy that becomes more evident with the progressive accumulation of lactate having surpassed the anaerobic threshold.
The consistency of these data in stages 1 to 3, and the small differences found between categories of body fat in stages 4 and 5, would permit the use of these results to estimate the oxygen uptake of children of both genders with high values of body fat similar to those studied in this analysis.
When comparing the relative oxygen uptake of the groups with different doses of exercise (0, 20, 40 minutes) at post-test with one-way ANOVA no differences were found for the stages 1 to 6 (ps > 0.40) thus showing no improvement in exercise economy (Table 5). The mean change observed in % body fat of the whole participating group was small (the whole group = − 1.1 ± 2.36), and insufficient to produce changes in economy with the different doses of activity; something that could be attributed to the short duration of the intervention program (4 months). However, there was a significant increment in the percentage of children who completed stages 4 to 7 of the test after the treatment period that could be due to familiarization with the test protocol or to an improvement in their CVF that permitted them to work at a higher intensity despite not showing changes in their submaximal VO2 (Table 6).
It is common to mention differences in VO2 max between boys and girls, but not at submaximal intensities, and it should be mentioned that these differences appear after puberty (Astrand and Rodahl, 1986; Bar-Or, 1983; Malina and Bouchard, 1991; McArdle, Katch el al., 2001). The first sign of the arrival of puberty in boys is usually an acceleration in the growth of the testicles and at the same time there may be the beginning of the growth of pubic hair, but this progresses slowly until the growth spurt. In girls, the growth of the breasts is usually the first sign, although it is sometimes preceded by the appearance of pubic hair. However menarche is the most commonly used sign by growth experts to characterize puberty. As already mentioned only 7 of the girls had experienced menarche (stage 5) at the end of the program and 8 boys had reached stage 2 and stage 3 of testicular development so it is reasonable to assume that maturation stage did not have an important effect in this sample.
Effort economy of the children as characterized by VO2 relative to body weight at the different stages was lower than that of adults when comparing the values obtained with those estimated for adults using the ACSM equation (2000: 303). It is well known that economy at submaximal loads below the anaerobic threshold improves with age up to adulthood (Bar –Or, 1983; Mc Ardle, Katch and Katch, 2001). Different authors have found that obese adult subjects have worse economy than leaner subjects, and better running economy has also been found among highly trained athletes (Bar-Or, 1983; Wasserman, Hansen et al., 1987). In our case the relative VO2 of the children was greater at all stages than that of the adults. There seemed to be a tendency towards a decrease in this difference as the intensity of the stages increased which could have been due to a better fitness level or the lesser number of children who were able to complete the higher loads. These differences should be taken into account to avoid underestimating the energy expenditure of physical activities.
When comparing relative VO2 in relation to the category of overweight, the values were slightly lower in the less obese children (≤ 41.4% body fat) at stages 4 and 5, 32.1 ± 3.1 and 34.1 ± 3.0 compared to 34.3 ± 3.1 and 36.6 ± 3.2 in the most obese. These differences, although slight, would seem to indicate a lower economy of effort in the most obese children that became more evident at the highest intensities.
When comparing VO2 at the different stages in absolute values (L/min), as could be expected because of a higher body weight, it was higher in the children with the greatest percentage of body fat. The higher absolute and relative VO2 values at submaximal loads illustrate the disadvantage of having to move a greater body mass, requiring a higher relative VO2 to move at the same velocity as their counterparts, which means a limitation when the loads increased during the last stages.
When referring to the usefulness of determinations of VO2 max with overweight and obese children, it would be better if we could say that the children gave their best effort in order to characterize their CVF. Unfortunately this was not always the case. The unfamiliar procedure and artificiality of walking on a treadmill with a steep slope, the collection of expired air which necessitated wearing a mouth piece and nose clip and its support or mask, made the test frankly uncomfortable.
With regard to the protocol used, the Bruce-type protocols have been criticized for providing very different load increases at the different stages and Balke-type protocols have been recommended in which the load increase at the different stages is more homogenous (Rowland 1999). This was the criterion used in the protocol originally proposed by Rowland (1993) and the ACSM (2000) for poorly fit children that recommends keeping the speed of the treadmill at 3 mph (4.83 km/h) and increasing the slope by 2% every 2 minutes once the warm-up is completed. Previous experience had proved that the starting load was too high for some of our overweight or obese children and led us to the inclusion of an additional load at 2.5 mph. (4.02 km/h) and 0% slope before the proposed warm-up at 3 mph. and 3% slope (Gutin et al., 2002). This was the criterion used in the current test protocol, in which the increase in oxygen uptake was consistent corresponding to an average of 2.6 mL/kg/min at each stage. It was precisely in this first phase of the warm-up that significant differences were found between the pre and post intervention tests compatible with a faster cardiovascular adjustment after the intervention. It is interesting that once the warm-up was completed the increases revealed in VO2 were similar at all stages.
The fact that estimation of oxygen uptake does not require equipment for analyzing samples of expired gases and therefore the children do not have to wear a series of devices like the mouth piece or mask could give them greater freedom and comfort to better reflect their capacity. Complaints about the mouth pieces or problems with the nose clips were common. Equally, being able to have normative values would permit the evaluation of CVF in a more economical fashion, and in less time, obtaining a better standardization of the loads compared with walking or running in the field as the speed would be controlled by the treadmill. Recording heart rate during the test and the physical activities would permit the establishment of a personalized relation between heart rate and oxygen consumption estimated from these values to calculate the energy cost of various activities.
Because of the children’s difficulty in walking uphill it may be useful to vary the speed of the protocol especially for children with a better fitness level or who are not overweight or obese so that they could do the test by running and eliminate the need to increase the slope; as increases in slope cause very acute local fatigue which is uncomfortable and causes the subjects to end the test before reaching their maximal VO2 values, as shown by the fact that in 190 tests (48.5%) the children stated that they had finished the test because of pain in their legs.
The RPE data recorded were not appropriate for this group of children given the high percentage of invalid data (79%) and of the remaining ones, the majority did not fulfil the requisites proposed by Börg (1974) as they were not related to the heart rate values when multiplied by 10. These observations coincide with what Bar-Or commented back in 1983 with regard to the difficulty children have in using this scale. If it is decided to use it with young aged subjects it may be helpful provide a more extensive description with examples and verify understanding so that the children can fully understand its meaning. However, preadolescents may be unable to utilize the abstract concept of a numerical scale of exertion (Piaget 1936).
To summarize, the values presented here can be used to estimate the relative VO2 of children similar to those studied, that is, in the same age range and overweight or obese, in practical situations walking on a treadmill like the protocol already mentioned, thus eliminating the need to use complicated gas analyzers. The elimination of the above-mentioned artefacts may facilitate their reaching a higher peak effort providing more accurate estimations of VO2. They also offer a criterion for the increase in VO2 which could be expected at the different stages of this stress test protocol which, due to the difficulties encountered for reaching a plateau during the test, could be used to decide if the effort has been maximal or not. If an individual performing a test increases less between stages than the increments reported here, that test could be considered maximal even if he or she does not reach a plateau of VO2.
Acknowledgments
This project data analysis has been carried out thanks to a research grant from the Salvador de Madariaga Programme from the Spanish Ministry of Education and Science- PR2006-0125.
Contributor Information
Agustín Meléndez-Ortega, Facultad de Ciencias de la Actividad Física y del Deporte-INEF-UPM. España.
Catherine Lucy Davis, Georgia Prevention Institute, Department of Pediatrics, Medical College of Georgia. USA.
Paule Barbeau, Georgia Prevention Institute, Department of Pediatrics, Medical College of Georgia. USA.
Colleen Ann Boyle, Georgia Prevention Institute, Department of Pediatrics, Medical College of Georgia. USA.
References
- ACSM. ACSM´s Guidelines for Exercise Testing and Prescription. 6th Ed. Philadelphia: Lippincot Williams & Wilkins; 2000. [Google Scholar]
- Astrand PO, Rodahl K. Textbook of Work Physiology: Physiological Bases of Exercise. 3rd Ed. New York: McGraw-Hill Book Company; 1986. [Google Scholar]
- Andersen LB, Harro M, et al. Physical fitness and clustered cardiovascular risk in children: a cross-sectional study. (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- Ariëns G, et al. The longitudinal development of running economy in males and females aged 13 and 27 years: The Amsterdam growth and health study. Euro J Appl Physiol. 1998:4. doi: 10.1007/s004210050239. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Balding J, et al. Peak Oxygen Uptake and Physical Activity in 11- to 16 year olds. Pediatric Exercise Science. 1990;2(4):349–358. doi: 10.1123/pes.2.4.349. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Welsman J. Exerc. Sports Sci. Rev. Vol. 22. ACSM; 1994. Assessment and interpretation of aerobic fitness in children and adolescents; pp. 435–476. [PubMed] [Google Scholar]
- Barbeau P, Gutin B, et al. Influence of physical training on plasma leptin in obese youths. Canadian Journal of Applied Physiology. 2003;28(3):382–396. doi: 10.1139/h03-028. [DOI] [PubMed] [Google Scholar]
- Bar-Or O. Pediatric Sport Medicine for the Practitioner; From Physiologic Principles to Clinical Applications. New York: Berlin, Heidelberg, Tokyo, Springer-Verlag; 1983. [Google Scholar]
- Börg G. Psychological aspects of physical activities. In: Larson LA, editor. Fitness, Health and Work Capacity: International standards for assessment. New York: Academic Press; 1974. pp. 141–163. [Google Scholar]
- Buono MJ, Roby JJ, et al. Predicting maximal oxygen uptake in children; Modification of the Astrand-Rhyming Test. Pediatric Exercise Science. 1989;1(1):278–283. doi: 10.1123/pes.1.3.278. [DOI] [PubMed] [Google Scholar]
- Buono MJ, Roby JJ, et al. Validity and reliability of predicting maximum oxygen uptake via field tests in children and adolescents. Pediatric Exercise Science. 1991;3(3):250–255. doi: 10.1123/pes.3.3.250. [DOI] [PubMed] [Google Scholar]
- Clausen JO, Borch-Jonen K, et al. Insulin sensitive index, acute insulin response, and glucose effectiveness in a population-based sample of 380 young healthy Caucasians. Analysis of the impact of gender, body fat, physical fitness, and life-style factors. Journal of Clinical Investigation. 1996;98:1195–1209. doi: 10.1172/JCI118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cureton KJ, Baumgartner TA, et al. Adjustment of 1-mile run/walk test scores for skinfold thickness in youth. Pediatric Exercise Science. 1991;3(2):57. doi: 10.1123/pes.3.2.152. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Howley ET. Substrate metabolism during exercise in children and the "crossover concept. Pediatric Exercise Science. 1999;11(1):12–21. [Google Scholar]
- Duncan GE, Mahon AD, et al. Plateau in oxygen uptake at maximal exercise in male children. Substrate metabolism during exercise in children and the "crossover concept. Pediatric Exercise Science. 1996;8(1):77–86. [Google Scholar]
- Endre T, Mattiasson I, et al. Insulin resistance is coupled to low physical fitness in normotensive men with a family history of hypertension. Journal of Hypertension. 1994;12:81–88. [PubMed] [Google Scholar]
- Eriksson KF, Lindgarde F. Poor physical fitness, and impaired early insulin response but late hyperinsulinemia, as predictors of NIDDM in middle-aged Swedish men. Diabetologia. 1996;39:573–579. doi: 10.1007/BF00403304. [DOI] [PubMed] [Google Scholar]
- Fagot-Campagna A, Pettit D, et al. Type 2 diabetes among North American children and adolescents: An epidemiological review and a public health perspective. Journal of Pediatrics. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- Freedson Ps, Goodman TL. Measurements of Oxygen Consumption. Pediatric Laboratory Exerecise Testing. Clinical Guide. T.W. Rowland Illinois: Human Kinetics; 1993. [Google Scholar]
- Gutin B, Owens S, et al. Weight-independent cardiovascular fitness and coronary risk factors. Arch Pediatr Adolesc Medicine. 1997 May;151:462–465. doi: 10.1001/archpedi.1997.02170420032005. [DOI] [PubMed] [Google Scholar]
- Gutin B, Barbeau P, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. Am J Clin Nutr. 2002;75:818–826. doi: 10.1093/ajcn/75.5.818. [DOI] [PubMed] [Google Scholar]
- Gutin B, Yin Z, et al. Relations of fatness and fitness to fasting insulin in black and white adolescents. J Pediatr. 2004;145:737–743. doi: 10.1016/j.jpeds.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Gutin B, Yin Z, et al. Relations of body fatness and cardiovascular fitness to lipid profile in black and white adolescents. Pediatric Research. 2005;58(1):78–82. doi: 10.1203/01.PDR.0000163386.32348.90. [DOI] [PubMed] [Google Scholar]
- Gutin B. Child Obesity Can Be Reduced with Vigorous Activity rather than Restriction of Energy Intake. Obesity. 2008;16:2193–2196. doi: 10.1038/oby.2008.348. [DOI] [PubMed] [Google Scholar]
- Harrell J, Mc Murray, et al. The multiple metabolic syndrome in young adolescents. Scientific Sessions Abstracts. 2000 Supplement 102 suppl 2. [Google Scholar]
- House of Commons. The stationery office limited. Third report of sessions 2003–2004. 2004:1. [Google Scholar]
- International Obesity Task Force. 2004 “www.iotf.org”. [Google Scholar]
- Jung AP, Nieman DC, et al. Prediction of maximal aerobic power in adolescents from cycle ergometry. Pediatric Exercise Science. 2001;13:167–172. [Google Scholar]
- Krahenbuhl G, Pangrasi R. Characteristics associated with running performance in young boys. Med. Sci. Sports Exerc. 1983;5:488. [PubMed] [Google Scholar]
- Krahenbuhl GS, Pangrasi RP, et al. Fractional utilization of maximal aerobic capacity in children 6 to 8 years of age. Pediatric Exercise Science. 1995;1(3):271–277. doi: 10.1123/pes.1.3.271. [DOI] [PubMed] [Google Scholar]
- Krekoukia M, Nassis GP, et al. Elevated total and central adiposity and low physical activity are associated with insuline resistance in children. Metabolism. 2007;56:206–213. doi: 10.1016/j.metabol.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Mader A, Heck H. A theory of the metabolic origen of anaerobic threshold. International Journal of Sport Medicine. 1986;7:45–65. [PubMed] [Google Scholar]
- Malina RM, Bouchard C. Growth, Maturation, and Physical Activity. Champaign, Ill: Human Kinetics; 1991. [Google Scholar]
- McArdle W, Katch F, et al. Exercise Physiology: Energy, Nutrition, and Human Performance. 5th Edition. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- Mc Veigh SK, Payne AC, et al. The reliability and validity of the 20-meter shuttle test as a predictor of peak oxygen uptake in Edinburgh school children, age 13 to 14 years. Pediatric Exercise Science. 1995;7(1):69–79. [Google Scholar]
- Mokdad A, Serdula M, et al. The spread of the obesity epidemia in the United States, 1991–1998. JAMA: The Journal of the American Medical Association. 1996;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- Noble BJ, Robertson RJ. Perceived Exertion. Champaign, Ill: Human Kinetics; 1999. [Google Scholar]
- Nyholm B, Mengel A, et al. Insulin resistance in relatives of NIDM patients: the role of physical fitness and muscle metabolism. Diabetologia. 1996;39:813–822. doi: 10.1007/s001250050515. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, et al. Centers for Disease Control and Prevention 2000 Growth Charts for the United States: Improvements to the 1977 National Center for Health Statistics Version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Piaget J. Delachaux et Niestlé. Neuschatel y París: 1936. La naissance de l’intelligence chez l’enfant. [Google Scholar]
- Pinhas-Hamiel O, Dolan L, et al. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. Journal of Pediatrics. 1996;128:606–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- Pitteti KH, Fernhall B, et al. Comparing two regression formulas that predict VO2 peak using the 20-m shuttle run for children and adolescents. Pediatric Exercise Science. 2002;14(2):125–134. [Google Scholar]
- Pitteti KH, Fernhall B, et al. A step test for evaluating the aerobic fitness of children and adolescents with mental retardation. Pediatric Exercise Science. 1997;9(2):127–135. [Google Scholar]
- Powell K, Blair S. The public health burdens of sedentary living habits: Theoretical but realistic estimates. Med. Sci. Sports Exerc. 1994;26:851–856. [PubMed] [Google Scholar]
- Reybrouck TM. The use of the Anaerobic Threshold. In: Bar-Or O, editor. Pediatric Exercise Testing. Advances in Pediatric Sport Sciences. Biological Issues. Volume Three. Champaign: Human Kinetics; 1989. pp. 131–149. [Google Scholar]
- Rivera-Brown AM, Rivera MA, et al. Applicability of criteria for VO2 max in active adolescents. Pediatric Exercise Science. 1992;4(4):331–339. [Google Scholar]
- Rivera-Brown AM, Rivera MA, et al. Achievement of VO2 max criteria in adolescent runners: effects of testing protocol. Pediatric Exercise Science. 1994;6(3):236–245. [Google Scholar]
- Rivera-Brown AM, Rivera MA, et al. Reliability of VO2 max in adolescent runners: A comparison Between Plateau Achievers and Nonachievers. Pediatric Exercise Science. 1995;7(2):203–210. [Google Scholar]
- Rosen C, Bouxsein M. Mechanisms of disease: Is osteoporosis the obesity of bone? Nature Clin Pract Rheumathol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Rowland T. Crusading for the Balke Protocol. Pediatric Exercise Science. 1999;11(3):189–192. [Google Scholar]
- Rowland TW. Aerobic Exercise Testing Protocols. In: Rowland TW, editor. Pediatric Laboratory Exercise. Testing Clinical Guide. Illinois: Human Kinetics; 1993. [Google Scholar]
- Rubin C, Capilla E, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siconolfi SF, Lasater TM, et al. Self-reported physical activity compared with maximal oxygen uptake. Am J. Epidemiol. 1985;122(3):452–457. doi: 10.1093/oxfordjournals.aje.a114068. [DOI] [PubMed] [Google Scholar]
- Strauss R, Pollack H. Epidemic increase in childhood overweight. JAMA: The Journal of the American Medical Association. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Jolly D, et al. Estimating the effect of energy imbalance on the effect of body weight in children. The American Journal of Clinical Nutrition. 83:859–863. doi: 10.1093/ajcn/83.4.859. [DOI] [PubMed] [Google Scholar]
- Tomassoni TL. Pediatric Laboratory Exercise Testing . Clinical Guide. Illinois: Human Kinetics; 1993. Conducting The Pediatric Exercise Test. [Google Scholar]
- Troiano R, Flegal K. Overweight children and adolescents: Description, epidemiology, and demographics. Pediatric. 1998;101:497–504. [PubMed] [Google Scholar]
- Turley KR, Rogers DM, et al. Maximal treadmill versus cycle ergometry testing children: Differences, reliability, and variability of responses. Pediatric Exercise Science. 1995;7(1):49–60. [Google Scholar]
- Wasserman K, Hansen JE, et al. Principles of Exercise Testing and Interpretation. Philadelphia: Lea & Febiger; 1987. [Google Scholar]
- Whipp BJ, Seard C, et al. Oxygen deficit-oxygen debt relationships and efficiency of anaerobic work. J Appl Physiol. 1970;28(4):452–456. doi: 10.1152/jappl.1970.28.4.452. [DOI] [PubMed] [Google Scholar]
- Whitaker R, Wright J, et al. Predicting obesity in young adulthood from childhood and parental obesity. NEJM. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Washington RL. Anaerobic Threshold. In: Rowland TW, editor. Pediatric Laboratory Exercise Testing: Clinical Guidelines. Champaign: Human Kinetics; 1993. pp. 115–129. [Google Scholar]
- WHO. Obesity and overweight. 2006. [Retrieved May 10, 2009]. from http://www.who.int/mediacentre/factsheets/fs311/en/index.html. [Google Scholar]
- WHO. Turning the tide of malnutrition: Responding to the challenge of the 21rst century. [Retrieved May 10, 2009];2000 from http://www.who.int/mip2001/files/2232/NHDbrochure.pdf. [Google Scholar]