Abstract
Alström syndrome (ALMS1) is a multisystemic disorder characterized by cone–rod dystrophy, hearing loss, obesity, insulin resistance and hyperinsulinemia, type 2 diabetes mellitus, dilated cardiomyopathy, and progressive hepatic and renal dysfunction.The cone-rod retinal dystrophy usually develops within a few weeks after birth. We examined a young boy with Alstrom by means of microperimetry MP-1 and optical coherence tomography (OCT) Spectral Domain.
Instead of the typical alterations observed in cone-rod dystrophies, the characteristics of the central foveal tissue suggest signs of retinal immaturity, with only a single layer of short thick cones and rods as well as immature short outer segments. High- speed/ high- resolution spectral domain OCT allowed for the first time a detailed analysis of retinal layers in a young patient with Alstrom Syndrome.
Keywords: alstrom syndrome, spectral domain optical coherence tomography (OCT), cyliopathies, retinal immaturity
Introduction
Alström syndrome (Online Mendelian Inheritance in Man [OMIN] 203800) is an autosomal recessive inherited disorder first described in 1959 by Alstrom et al.1 The gene, ALMS1, was mapped to chromosome 2p13, and several disease causing mutations have been identified.2 Cardinal feature of this disorder are cone–rod dystrophy, hearing loss, obesity, insulin resistance and hyperinsulinemia, type 2 diabetes mellitus, dilated cardiomyopathy, and progressive hepatic and renal dysfunction3.
The ALMS1 gene encodes a protein lacking previously described domains. The protein is found primarily in centrosomes and basal bodies of ciliated cells, suggesting a function in cilia formation, maintenance and function.4
Ocular manifestations occurring in the first years of life include nystagmus and photophobia with diminished visual acuity. Narrowing of the retinal vessels, chorioretinal atrophy, bone spiculae pigmentary changes, and optic atrophy are seen in the fundus, and posterior subcapsular cataracts may be present.5–6
The present study focuses on the evaluation of retinal ultrastructure in a young boy with Alstrom by means of microperimetry and high- resolution optical coherence tomography (OCT) Spectral Domain.
Case Report
A 5-year-old boy was referred to the Department of Ophthalmology of University of Rome “La Sapienza”, Polo Pontino, with a complaint of progressive vision loss, with decreasing visual acuity since birth. The patient had a normal gestation and birth and no medical record of inflammatory or other acute ocular disease. He was obese and had dilated cardiomyopathy. Familial medical history was unremarkable.
Informed consent was obtained from the parents for further medical and genetic investigations. Genomic DNA was extracted from peripheral blood for mutation screening for Alstrom Syndrome was performed. The homozygous mutation, 8938C>T Gln2980Ter, was found on exon 10 in ALMS1, which confirmed ALMS.
The patient underwent a complete ophthalmologic evaluation including assessment of best corrected visual acuity (BCVA), slitlamp biomicroscopy examination, indirect ophthalmoloscopy, visual field examination, color vision testing, electoretinogram (ERG) and visual evoked potentials (VEP), microperimetry (MP-1 Nidek Technologies, Italy) and high- resolution OCT (OCT Spectralis, Heidelberg Engeneering, Germany).
The best corrected visual acuity was 0.52 logMAR in both eyes (+2.00 = +1.00/120 RE; +3.00 = +1.00/45 LE) and there was both photophobia and pendular nystagmus. Fundus examination revealed pale optic discs with drusen, cone-rod dystrophy, and narrowing of the retinal vessels in both eyes.
Visual fields were examined with Goldmann kinetic perimetry using standardized targets III:4e and I:4e with white light stimulus. The patient had severely constricted visual fields to approximately 20° and 10° with the III:4e target and to 10° and 5° with the I:4e target in right eye and left eye respectively. Color vision was tested using the Farnsworth D-15 color vision test. Patient had confusion regarding color discs that were close together, which is not considered significant, and the lines in both eyes remained along the outside of the circle.
The mixed cone-rod electroretinogram (ERG), was reduced in both eyes. The visual evoked potential (VEP) amplitudes were reduced in the right eye (RE) and normal in the left eye (LE). Microperimetry demonstrated a central absolute scotoma in the RE and two paracental scotomas in the LE. Retinal sensitivity was 14,8 dB in the RE and 16 dB in the left eye. Fixation behaviour was relative unstable in the both eyes (as the 77% of the fixation points were inside the 4° diameter circle in the RE and 79% of the fixation points were inside the 4° diameter circle in the LE).7
High definition spectral domain optical coherence tomography scans showed a slight thinning of the central retina and persistent inner retinal layers (similar to paramacular scans) within the fovea. The connecting cilium in the outer retina was absent, and there was no alteration of the retinal pigment epithelium and Bruch’s membrane.
Discussion
High- speed and high- resolution spectral domain OCT allowed for the first time a detailed analysis of retinal layers in a young patient with Alstrom Syndrome.
Instead of the typical alterations observed in cone-rod dystrophies, the characteristics of the central foveal tissue (figure 1 and figure 2) suggest signs of retinal immaturity, with only a single layer of short thick cones and rods as well as immature short outer segments.8
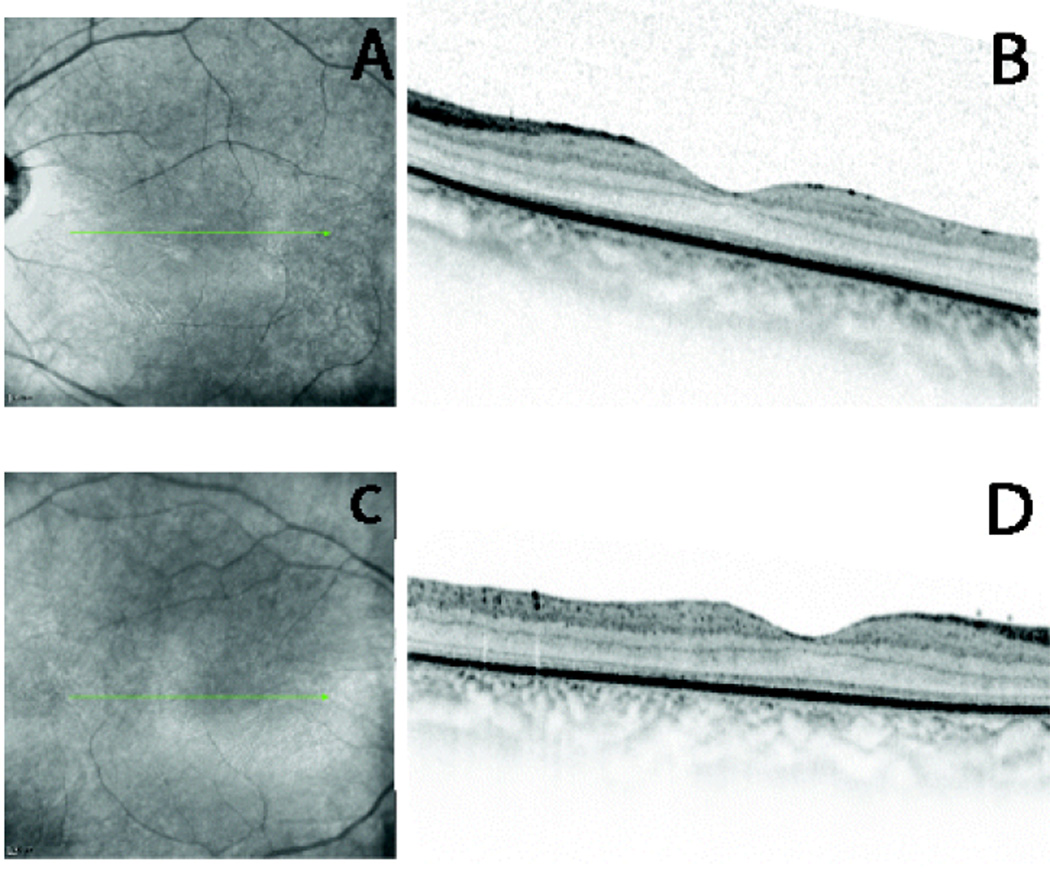
Figure 1.
(A and C) fundus photography of LE and RE respectively, the arrows indicate the direction of scans; (B and D) high definition spectral domain optical coherence tomography horizontal scans (OCT Spectralis, Heidelberg Engeneering, Germany) showing retinal immaturity both in LE and RE respectively.
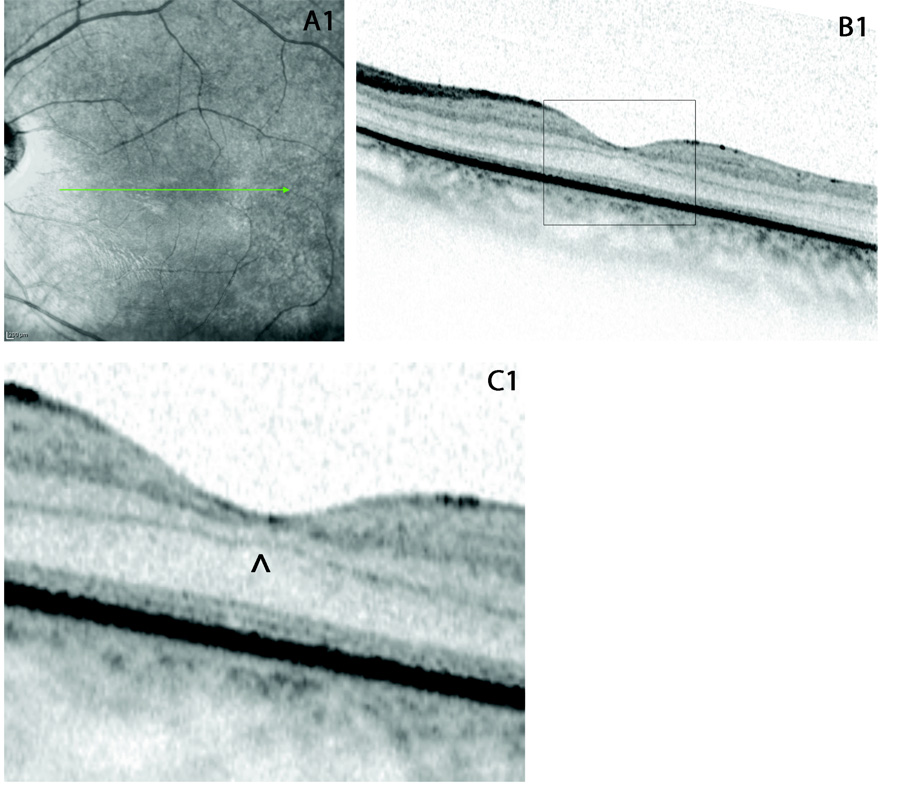
Figure 2.
(A1) fundus photography of LE, the arrow indicate the direction of scan; (B1) high definition spectral domain optical coherence tomography horizontal scan (OCT Spectralis, Heidelberg Engeneering, Germany), the black rectangular indicates the detail that has been enlarged in C1; (C1) enlarged detail of the horizontal OCT scan in the LE: the central retina exhibits the persistence of inner retinal layers (similar to paramacular scans) within the fovea, indicated by the ^; the connecting cilium in the outer retina is absent, and there is no alteration of the retinal pigment epithelium and Bruch’s membrane.
On a superficial analysis, our data seems to contradict the results of the necropsy findings of Sebag and coworkers5. They found that the retina had markedly hypocellular ganglion cell, inner and outer nuclear cell layers, rod and cone outer segments were absent, the retinal pigment epithelium (RPE) was disrupted throughout and there was optic nerve atrophy and giant drusen of the optic disc in both eyes.
This discrepancy of results may be attributed to the progressive nature of retinal degeneration in AS. Furthermore, they state that histopathologic findings in this disease differ from the histopatological characteristics of primary retinitis pigmentosa, and this could be explained by our findings. In fact, the in vivo imaging of retinal derangement by OCT, suggest that, although the ocular features of this syndrome have been always explained as a cone-rod dystrophy, AS is characterized by the arrest of macular development and abnormal persistence of the early retinal structural organization. This finding could explain why these patients experience a earlier and more severe visual loss than typical cone-dystrophies. Our results are limited to one case only, but shed new light on the ocular characteristics of this syndrome and can serve as a future reference to estabilish its pathophysiology.
Footnotes
There is no financial conflict of interest in the subject matter of the manuscript, neither any commercial or proprietary interest.
References
- 1.Alstrom CH, Hallgren B, Nilsson LB, Asander A. Retinal degeneration combined with obesity, diabetes mellitus, and neurogenous deafness: a specific syndrome (not hitherto described) distinct from the Laurence- Moon- Bardet- Biedl syndrome: a clinical endocrinological and genetic examination based on a large pedigree. Acta Psychiatr Neurol Scand. 1959;34(1) suppl 129:1–35. [PubMed] [Google Scholar]
- 2.Malm E, Ponjavic V, Nishina PM, Naggert JK, Hinman EG, Andréasson S, Marshall JD, Möller C. Full-field electroretinography and marked variability in clinical phenotype of Alström syndrome. Arch Ophthalmol. 2008 Jan;126(1):51–57. doi: 10.1001/archophthalmol.2007.28. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JD, Beck S, Maffei P, Naggert JK. Alström Syndrome. Eur J Hum Genet. 2007;15(12):1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- 4.Hearn T, Spalluto C, Phillips VJ, et al. Subcellulare localization of ALMS1 supports involment of centrosomes and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54(5):1581–1587. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 5.Sebag J, Albert DM, Craft JL. The Alström syndrome: ophthalmic histopathology and retinal ultrastructure. Br J Ophthalmol. 1984 Jul;68(7):494–501. doi: 10.1136/bjo.68.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell-Eggitt IM, Clayton PT, Coffey R, Kriss A, Taylor DS, Taylor JF. Alström syndrome. Report of 22 cases and literature review. Ophthalmology. 1998 Jul;105(7) doi: 10.1016/S0161-6420(98)97033-6. 1274-80.ù. [DOI] [PubMed] [Google Scholar]
- 7.Fujii GY, de Juan E, Jr, Sunness J, Humayun MS, Pieramici DJ, Chang TS. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109:1737–1744. doi: 10.1016/s0161-6420(02)01120-x. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickson AE, Youdelis C. The morphological development of the Human Fovea. Ophthalmology. 1984;91:603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]