Abstract
OBJECTIVE
Diindolylmethane, a natural product from cruciferous vegetables, has been shown to be a dietary component that has inhibitory effects on some tumors (e.g., laryngeal papilloma). However, current evidence to support its safety is based on adult humans or mature animals. There is little to show its safety in children. This study is designed to asses safety in the young rat model
STUDY DESIGN
Prospective Controlled Animal Study.
METHODS
40 rats were separated into 4 treatment groups of 10 rats each, based on the amount of study drug they received in their daily food: 1. Immature rats fed low dose DIM, which is our proposed treatment dose (2.0mg/kg/day). 2. Immature rats fed high dose DIM (20.0mg/kg/day). 3. Immature rats fed no DIM (control). 4. Adult rats fed high dose DIM (20.0mg/kg/day). At the conclusion of the study we collected blood to compare serum chemistries and vitamin D levels, and harvest organs to observe for any gross or histological changes between the groups. Statistical methods involved one-way ANOVA and pairwise comparisons with Tukey’s multiple comparison adjustment.
RESULTS
Although our numbers do not allow for statistical significance, there was no appreciable difference in rat weights between the immature groups, nor was there appreciable difference between serum chemistries, or gross or histological examination of liver, kidney, or bone.
CONCLUSIONS
Diindolylmethane seems to have no adverse affects on the rat even when given in doses 10x what we propose to be therapeutic. This adds evidence to the safety of this drug in the pediatric population as a treatment option for recurrent respiratory papilloma.
Introduction
Cancer chemoprevention/therapy is a promising approach to fight tumors. Unfortunately, currently used agents have significant side-effects. It is a well accepted fact that many human cancers could be prevented or reduced by changing lifestyle, including dietary modification. 3,3′-diindolylmethane (DIM), a natural product from cruciferous vegetables, has been shown to be a dietary component that has such inhibitory effects on some cancers as well as recurrent respiratory papilloma (RRP). However, current evidence to support its safety is mostly based on adult human or mature animal studies. There is minimal evidence in the literature to show its safety in children, in whom this disease process can be the most devastating. In this study, we conducted a safety feasibility experiment in sexually immature rats by collecting blood and tissue samples to screen for possible side effects. Our goal in this study is to determine if oral DIM is a safe drug for young rats, and if DIM poses a greater risk in immature rats when compared to mature ones. By accomplishing this, we will be able to fill a gap in the literature regarding the safety of this drug in this patient population.
Materials and methods
Chemicals
DIM powder was obtained from BioResponse® (Boulder, CO). Independent laboratory analysis by Eurofilms® via High Performance Liquid Chromatography (HPLC) found the DIM to be 99.4% pure.
Animals
Approval for animal use in research was obtained from our animal oversight committee, the Institutional Animal Care and Use Committee (IACUC). 40 Sprague-Dawley rats were purchased from Charles River Inc. in Boston, MA. 30 Rats were sexually immature at 21 days old, and 10 were mature at 60 days old. The animal housing facility was the Center for Advanced Biomedical Research (W-Bldg) at Boston University School of Medicine. To track individual rats within a cage, a rat in each cage had a small piece of their ear clipped.
Design
Rats were separated into four groups based on age and DIM concentration in their food. All rats underwent a 24 hour acclimation period prior to the start of the study. Immature rats were divided into 3 different treatment groups of 10 each: (1) low-dose DIM (2.0 mg/kg/day) (current maximum human dose); (2) high-dose DIM (20.0 mg/kg/day); and (3) no DIM as a control. The adult group was given a high dose regimen to serve as a control to the immature group in the event that there were biochemical/histological findings in the young groups. There were 5 male and 5 female rats in each of these 4 groups. The rats were housed 2 or 3 per cage (same sex in each cage), and maintained at 12-h light/dark cycle. Tap water was available ad lib throughout the study, and the food was given after calculating the amount of required food per gram of rat (see next section).
Study food calculations
Prior to initiation of the study, we calculated the feeding requirements of both adult and immature rats. We measured how much food was consumed by each cage per day, over 3 days, then divided that by the total rat weight for the cage. This provided a reasonable estimate of how much was consumed per gram of rat. We calculated that the rats were required 15% of their bodyweight/day (including wastage).
Study food creation
We decided to put all of the study drug into 1/5th of each day’s food requirement, giving the other 4/5th’s as normal preformed pellet food. We incorporated DIM meal mix 18% protein rodent diet from Harlan Teklad Global diets (Crossett, AR) into ratios that would give us the per gram requirement. The soft meal mix was then formed into pellets by using a standard cake funnel and allowed to dry via a circulating fan away from direct sunlight. In this manner we were able to ensure that the low and high dose groups would consume 2.0 and 20.0 mg/kg/day respectively.
Study Food administration
Since drug administration is weight based, at the initiation of the study we weighed all the rats in order to administer the correct amount of study drug. This ensured that all food was consumed, allowing us to achieve a reasonable daily concentration of DIM in the rats. Rats were reweighed every 2–3 days and the new food requirements were recalculated. Enough food was left in the cages to last until the next weigh/feeding day.
Blood collection and analysis
2 test rats from group 2, and 2 from group 3 had a blood sample collected via the tail vein on day 7, after fasting overnight. This was done as a “dry run” to ensure that we were able to collect samples and to ensure the reliability of our laboratory in performing the required analysis. On day 32, all 40 rats were euthanized, after a 24hr fast, via inhalation of CO2 and blood samples were collected. 0.5 c.c. serum was made from each blood sample, and then sent to the Veterinary Medical Center of Michigan State University (Lansing, MI) for comprehensive chemistry profiles and endocrinology testing.
Bone density analysis
After euthanasia, the knee joint was removed and embedded in glycol methacrylate. The samples were sectioned on a rotary microtome with H&E staining for microscopic observations.
Histopathology
Gross observation was conducted for liver and kidneys under microscope in order to identify any variances. The tissue samples from these organs were then processed as per routine procedures and H&E staining in histopathology for microscopic examinations.
Statistical analysis
The 6 subgroups (4 treatment groups and 2 different sexes) were analyzed separately, and then compared to each other to determine any significant difference. The data were compared using one-way ANOVA and all pairwise comparisons with Tukey’s multiple comparison adjustment. When outliers or other non-normality was indicated, simple transformation (eg. Logarithmic) was examined.
Results
General
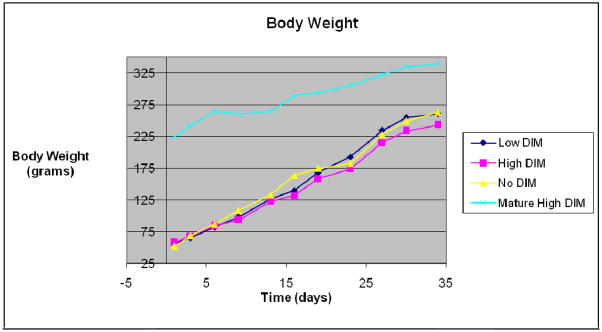
All 40 rats exhibited normal growth and development throughout the 40 day study period, as evidenced by equivalent growth between groups (Figure 1). There were no visible signs of distress or conflict during the entire study period. All rats tolerated the study protocol.
Figure 1.
Rat weights during study
Blood chemistries
Initially, we conducted chemistry tests with a comprehensive metabolic profile (total of 30 items tested) in a total of 8 serum samples collected at day 7 from 4 rats in groups 2 (High DIM) and 3 (no DIM). There was no statistical difference between the two groups (N=8, P >0.1).
On day 32, we collected serum samples from all 40 rats, after a 24hr fast, immediately after they were euthanized. We repeated the same chemistry tests and statistical analysis on these 40 samples (Table 1). Differences in means for continuous variables (untransformed and log-transformed to adjust for non-normality) across the four groups were assessed using ANOVA, and Fisher’s Exact tests were used to compare the distribution of the categorical variables across the four treatment groups. A Hierarchical testing approach was implemented. We first looked at overall difference between the four groups, and if the difference was significant at 0.05 level, then we preceded with analysis of overall difference within young animals, and difference between young and adult animals. If the overall difference within young animals was significant, we tested differences in high dose DIM group versus control and low dose DIM group versus control (Table 1).
Table I.
Chemistry test results (N=40, with 30 test items) on day 32
| Tests | Chemistry Tests and Results (P value) | ||||
|---|---|---|---|---|---|
| overall | young | adult vs. young | |||
| overall | H vs. C | L vs. C | |||
| urea nitrogen | * | ||||
| creatinine | * | ||||
| Na+ | |||||
| K+ | |||||
| Cl− | |||||
| NaK ratio | |||||
| TCO2 | |||||
| anion gap | |||||
| osmol. cal | |||||
| Ca+ total | |||||
| phosphorus | ** | ** | |||
| magnesium | |||||
| iron | * | ||||
| protein total | ** | ** | |||
| albumin | * | ||||
| globulin | * | ** | |||
| glucose | |||||
| amylase | |||||
| ALP | * | ** | |||
| total bilirubin | * | ||||
| direct bilirubin | |||||
| indir. bilirubin | |||||
| ID SDH | |||||
| ALT | |||||
| AST | * | ** | |||
| CK | ** | ** | |||
| total cholest. | * | ||||
| hemolysis | |||||
| chem.. icterus | |||||
| chem. lipemia | |||||
Note:
P <0.05;
P <0.001, all unmarked items/tests = P >0.05 H vs C = high-dose DIM vs control; L vs C = low-dose DIM vs control
The analysis yielded significant overall differences in both untransformed and log-transformed values of Phosphorus (p=0.008), Total Protein (p=0.008), Globulin (p=0.01), and Total Cholesterol (p=0.04); and in only log-transformed ALP (p=0.02), AST (p=0.01) and CK (p=0.006). All observed differences were due to difference between young and adult animals. All overall difference tests within young animals were not significant (Table 1). The analysis detected no overall significant difference in Urea Nitrogen, Creatinine, Sodium, Potassium, Chloride, Na/K Ratio, TCO2, Anion Gap, Osmolarity Calc, Total Calcium, Magnesium, Iron, Albumin, Glucose, Amylase, Total Bilirubin, Direct Bilirubin, Indirect Bilirubin, ID (SDH), ALT, Hemolysis, Chemistry Icterus and Chemistry Lipemia.
Body and organ weights
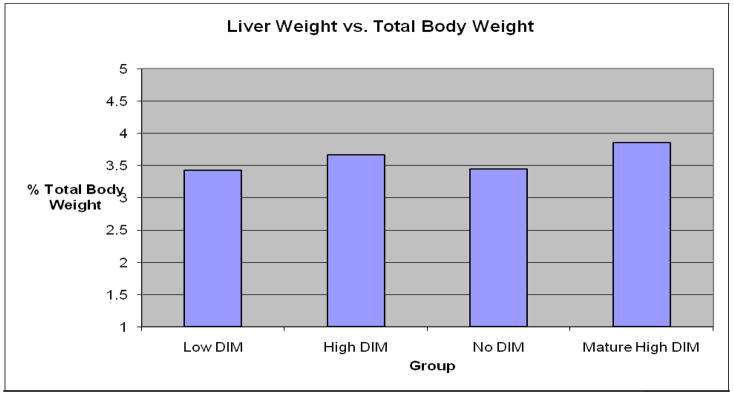
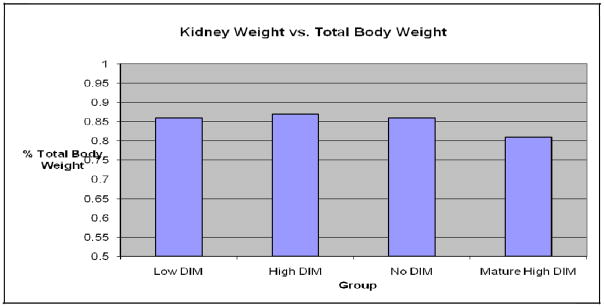
There were no significant differences in diet consumption or weight gain between the 3 young rat groups over the 32-day period (N=40, P >0.1, Fig. 1). There were also no treatement –related effects on liver as well as ratio of body and liver weight (liver somatic index, LSI), in either sex (P >0.5, Fig. 2). The same was the true on kidney and the ratio of body and kidney weight (kidney somatic index, KSI), (N=40, P >0.1, Fig. 3).
Fig. 2.
Liver somatic index (LSI) at day 32
Fig. 3.
Kidney somatic index (KSI) at day 32
Gross and microscopic pathology
No significant gross differences between these 4 groups of either sex at day 32 was noted upon necropsy (Fig. 4) or following histopathology. Most notably, no effects were seen in DIM-metabolized organs such as kidney and liver. All of the tissues and organs showed a normal structure and appearance. Neither toxicity nor other abnormality was indicated in either low or high DIM dose groups, as compared to the controls.
Figure 4.
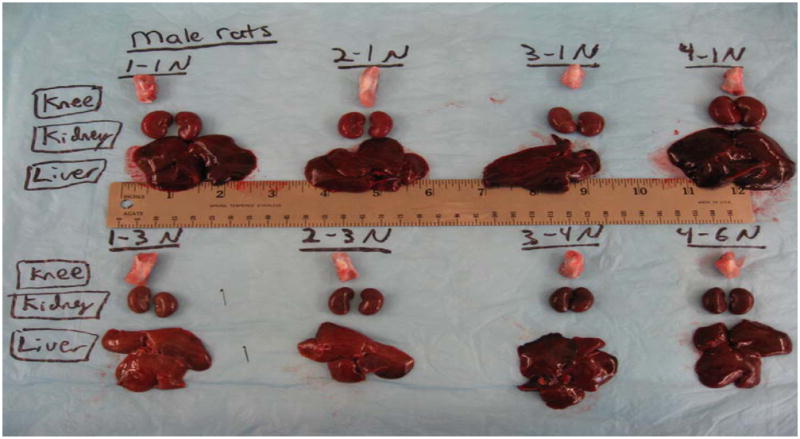
Gross examination of organs top to bottom: Knee, Kidney, Liver
Bone density
Histomorphometric analysis of decalcified cancellous and cortical bone from the DIM-treated young rats showed normal structures and appearance. Our microscopic results indicated that, in comparison to the control group, the DIM diets were not associated with any additional adverse effects on bony density, structure, or turnover. Additionally, we measured 25-Hydroxyvitamin D levels of four rats from the four groups on day 32 to asses for any other clues that new bone creation and resorption was affected. We did not discover any major difference between these 4 groups (P >0.1, Table 2).
Table II.
Results of 25-Hydroxyvitamin D (N=16) on day 32
| 25-Hydroxyvitam D | |||||
|---|---|---|---|---|---|
| Rat | Sex | Group1 | Group2 | Group3 | Group4 |
| 2R | male | 29 | 40 | 30 | 49 |
| 2N | male | 30 | 33 | 31 | 38 |
| 4R | female | 46 | 41 | 38 | 42 |
| 4N | female | 35 | 36 | 48 | 32 |
| mean | 35 | 37.5 | 36.75 | 40.25 | |
Discussion
Recurrent Respiratory Papillomatosis (RRP) is a noncurable resiliant disease that inturn requires vigilant survailance and treatment. The only universlly accepted method of managing RRP is surgical resection when airaway or voice morbidities necessitate interveation. Cold knife, microdebridement, and the ablative Carbon Diaxoide (C02) laser have been long accepted modalities. Unfortunately, there is the uwanted side-effect of scar tissue formation with the multitude of surgiers required in this population. More recently, angiolytic laser therapy via the 532-nm potassium titanyl phosphate (KTP)[1] and pulsed dye laser (PDL)[2] have been investigated as a method to manage papilloma by destroying it’s blood supply, which also confers the benefit of decreasing the resultant scarring that develops.
Medical treatments continue to be explored with hopes of discovering ways to prolong the intervals between surgeries, which are often inevitable. Hormonal therapies include 3,3′-diindolylmethane (DIM) and Indole-3-carbinol (I3C) which are aimed at altering the effects of estrogens on papillomas, no serious side affects have been noted to date. Anti-viral drugs (e.g. Cidofovir) are also at time used to directly target the viral burden of HPV at sites of papilloma growth, currently no common serious side-affects are noted[3]. Another studied method is by alteration of the patients immune system with Interferon alpha (IFN-a), this method had reserved for severe cases and also been connected with neurological and developmental abnormalities in children. It is important to understand that preventative measures attack the different causative factors of RRP, and therefore are not mutually exclusive. On the contrary, a multi-dimensional medical and surgical regimen may provide the best results in these patients.
DIM has been widely studied for its anti-carcinogenic properties. DIM and its precursor I3C are naturally occurring plant alkaloids formed by the hydrolysis of indole glucosinolate, found mainly in cruciferous vegetables such as broccoli and Brussels sprouts [4]. Some past studies have focused on I3C as the active agent, but new evidence leans towards DIM as the true active compound responsible for the antitumor activities, this coupled with reports of toxicity associated with oral use of I3C, which relate to unfavorable enzyme induction, are pointing toward DIM as the agent of choice [5].
Although the exact manner in which DIM may produce antitumor effects has not been defined, numerous studies have suggested specific mechanisms as to how DIM may be beneficial for specific tumors, most notably breast and prostate [6,7]. A recent study by Chinnakannu et al. [8] demonstrated that DIM inhibited the progression of synchronized prostate cancer cells from G(1) to S phase by induction of p27(Kip1) and down-regulation of the androgen receptor pathway, also that it inhibits proteasome activity in S phase, leading to apoptosis in certain cells. Similar activity has been noted in breast tissue by Howells et al [9]. S-phase retardation and mitotic delay has also been linked to topoisomerase II alpha-catalyzed ATP hydrolysis by DIM [10]. Another study by Denger et al. suggests that since it is known that DIM is an antagonist of the aryl hydrocarbon receptor (AhR), this modulation may be beneficial because the activation of cyclooxygenase-2 (COX-2) expression by AhR ligands may contribute to inflammation and tumorigenesis [11]. New reports are starting to show that DIM may also be beneficial in certain lung cancers [12].
The mechanism of action in regard to RRP has been less specific. A leading theory revolves around the estrogen metabolism modulation effects of DIM. Prior studies in humans and rats have noted that cruciferous vegetables and I3C, increase metabolism for estrogen creating beneficial estrogens (2-hydroxy estrogens), which increases antioxidant activity. At the same time reduces 16-hydroxy estrogens, which are not antioxidants and can potentially cause cancer [13, 14].
This study was designed to better understand the implications of using DIM in immature rats and eventual use in the pediatric population. There is currently minimal data regarding the use of DIM in the treatment of RRP. Prior safety studies have mainly focused on older rats and/or humans. A study by Leibelt, et. al compared the safety of DIM to I3C in rats that were 5 weeks old that showed no gross of histological adverse effect or toxicity, but did note that DIM is less efficacious than IC3 in inducing CYP in rats [15]. Human studies have shown a dose of up to 200mg is well tolerated and increasing the dose did not add anything to the pharmacokinetics [16].
Prior reports of use of this drug in pediatric population were limited to a case report where an 8 year old girl reported disappearance of RRP over a 27mo period with the use of intralesional and intravenous Cidofovir in association with indole-3-carbinol [17], and a prospective trial of 18 adults and children with RRP, which showed 33% of the subjects resulting in cessation of papilloma growth, and 33% with decreased papilloma growth [18]. No previous studies have looked at specifically DIM’s safety in the pediatric population.
Clinical chemistry panels failed to uncover any significant differences between the 3 young rat groups. Statistical differences in some tests (e.g., phosphorus, AST, ALT) were found between the young rat groups and the adult group. This, however, corresponds to some variation that would be expected in the chemistries between young and older animals.
Conclusion
The data from this study indicated that DIM is safe, with no significant side-effects found, even with DIM at a dose 10 times higher than that currently used in practice. It further confirmed results and safety reported from previous studies in adult humans and the adult animal model. There was a concern that in some cases, DIM may influence levels of vitamin D or hormones, which in turn could affect bone density. In this study, no effect on bone density or Vitamin D levels was observed following DIM exposure. Additionally, histopathological examination did not demonstrate any changes in liver or kidney. This study demonstrated that DIM is a relatively non-toxic compound that seems to be safe for use in the pediatric population. Our results supported its clinical application in the author’s ongoing child papilloma study, and allowed us to begin patient recruitment.
Acknowledgments
Special thanks to Lindsay Miller, MS (Boston University School of medicine) for help in the preparation the manuscript.
Financial Support from NIH Grant # 5R01DC008287-02
Footnotes
Work Performed at Boston University school of Medicine, Boston, Ma, USA
Presented at Triologic Society Eastern Section meeting 2009, January 24, 2009 Boston, Ma, USA
References
- 1.Zeitels SM, Akst LM, Burns JA, et al. Office-based 532-nm pulsed KTP laser treatment of glottal papillomatosis and dysplasia. Ann Otol Rhinol Laryngol. 2006;115(9):679–85. doi: 10.1177/000348940611500905. [DOI] [PubMed] [Google Scholar]
- 2.Mouadeb DA, Belafsky PC. In-office laryngeal surgery with the 585nm pulsed dye laser (PDL) Otolaryngol Head Neck Surg. 2007;137(3):477–81. doi: 10.1016/j.otohns.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Bielecki I, Mniszek J, Cofaa M. Intralesional injection of cidofovir for recurrent respiratory papillomatosis in children. Int J Pediatr Otorhinolaryngol. 2009 Feb 2; doi: 10.1016/j.ijporl.2009.01.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Bradfield CA, Bjeldanes JF. Structure-activity relationships of dietary indoles: A proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987;21:311–322. doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- 5.Bradlow HL. Review. Indole-3-carbinol as a chemoprotective agent in breast and prostate cancer. In Vivo. 2008;22(4):441–5. [PubMed] [Google Scholar]
- 6.Gong, et al. 3,3′-Diindolylmethane is a novel mitochondrial H(+)ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Research. 2006;66(9):4880–7. doi: 10.1158/0008-5472.CAN-05-4162. [DOI] [PubMed] [Google Scholar]
- 7.Zhao YY, Zhou L, Pan YZ, et al. 3,3-diindolylmethane enhances the inhibitory effect of idarubicin on the growth of human prostate cancer cells. Zhonghua Yi Xue Za Zhi. 2008;11;88(10):661–4. [PubMed] [Google Scholar]
- 8.Chinnakannu K, Chen D, Li Y, et al. Cell Cycle-Dependent Effects of 3,3′-diindolylmethane on proliferation and apoptosis of Prostate Cancer Cells. JCell Physiol. 2008 doi: 10.1002/jcp.21650. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howells LM, Gallacher-Horley B, Houghton CE, et al. Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol Cancer Ther. 2002;1:1161–72. [PubMed] [Google Scholar]
- 10.Gong Y, Firestone GL, Bjeldanes LF. 3,3′-diindolylmethane is a novel topoisomerase IIalpha catalytic inhibitor that induces S-phase retardation and mitotic delay in human hepatoma HepG2 cells. Mol Pharmacol. 2006;69 (4):1320–7. doi: 10.1124/mol.105.018978. [DOI] [PubMed] [Google Scholar]
- 11.Degner SC, Papoutsis AJ, Selmin O, et al. Targeting of Aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3-diindolylmethane in breast cancer cells. J Nutr. 2009;139(1):26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassie F, Anderson LB, Scherber R, et al. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67(13):6502–11. doi: 10.1158/0008-5472.CAN-06-4438. [DOI] [PubMed] [Google Scholar]
- 13.Newfield L, Goldsmith A, Bradlow HL, et al. Estrogen metabolism and human papillomavirus-induced tumors of the larynx: chemoprophylaxis with indole-3-carbinol. Anticancer Res. 1993;13(2):337–41. [PubMed] [Google Scholar]
- 14.Auborn K, Abramson A, Bradlow HL, et al. Estrogen metabolism and laryngeal papillomatosis: a pilot stud on dietary prevention. Anticancer Res. 1998;18(6B):4569–73. [PubMed] [Google Scholar]
- 15.Leibelt D, Olaf RH, Hedstrom KA, et al. Evaluation of Chronic dietary esposure to indole-3-Carbinol and absorption-enhanced 3,3-Diindolylmethane in Sprague-Dawley Rats. Toxicol Sci. 2003;74:20–2. doi: 10.1093/toxsci/kfg103. [DOI] [PubMed] [Google Scholar]
- 16.Reed GA, Sunega JM, Sullivan DK, et al. Since –dose pharmacokinetics and tolerability of absorption-enhanced 3-3′-diindolylmethane in healthy subjects. Cancer Epidemiol Biomarkers Prev. 1008;17(10):2619–24. doi: 10.1158/1055-9965.EPI-08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bilderling G, Bodart E, Lawson G, et al. Successful use of intralesional and intravenous Cidofovir in association with indole-3-carbinol in an 8 year-old girl with pulmonary papillomatosis. J Med Virol. 2005;75:332–335. doi: 10.1002/jmv.20275. [DOI] [PubMed] [Google Scholar]
- 18.Rosen CA, Woodson Bryson PC. Indole-3-carbinol for recurrant respiratory papillomatosis:long-term results. J Voice. 2004;200418:248–253. doi: 10.1016/j.jvoice.2003.05.005. [DOI] [PubMed] [Google Scholar]