Abstract
Invertebrate herbivores frequently face growth rate constraints due to their high demands for phosphorus (P) and nitrogen (N). Temperature is a key modulator of growth rate, yet the interaction between temperature and P limitation on somatic growth rate is scarcely known. To investigate this interaction, we conducted a study on the somatic growth rate (SGR) of the cladoceran Daphnia magna, known to be susceptible to P-limitation. We determined the SGR across a broad range of dietary P content of algae (carbon (C):P ratios (125–790), and at different temperatures (10–25°C). There was a strong impact of both temperature and C:P ratio on the SGR of D. magna, and also a significant interaction between both factors was revealed. The negative effect of dietary C:P on growth rate was reduced with decreased temperature. We found no evidence of P limitation at lowest temperature, suggesting that enzyme kinetics or other measures of food quality overrides the demands for P to RNA and protein synthesis at low temperatures. These findings also indicate an increased risk of P limitation and thus reduced growth efficiency at high temperatures.
Keywords: Algae, Aquatic, Food quality, Freshwater, Stoichiometry
Introduction
A large number of experiments and field studies have demonstrated that consumers, most notably herbivores, can face dietary phosphorus (P) limitation (Sterner and Elser 2002). In essence, this effect is commonly due to a high carbon (C):P ratio in autotrophs relative to consumers demands. This implies that there will be an excess intake of C that needs to be disposed off, and which translates into reduced growth efficiencies (Hessen and Anderson 2008). Moreover, P deficiency may constrain the synthesis of ribosomes, thereby reducing protein synthesis and consequently somatic growth (Elser et al. 2000).
Most experiments and observations related to nutrient limitation have been conducted in a temperature range between 15 and 25°C, and there is scarce and somewhat mixed evidence for the relevance of stoichiometric regulation at low temperatures. For autotrophs, the content of P and N relative to C generally increase with increasing latitude, which is interpreted as a temperature response (Reich and Oleksyn 2004; Kerkhoff et al. 2005; Lovelock et al. 2007). Although the data are scarcer, the same tendency seems to also hold true for metazoans (Moore et al. 1996; Woods et al. 2003). Cold-adapted ectotherms show higher cell-specific levels of P and rRNA than individuals of the same species living under higher temperatures (Woods et al. 2003). This has been interpreted as a compensatory response to a reduced efficiency of protein synthesis at low temperatures, i.e. more ribosomes are needed to maintain a given protein synthesis rate at low temperatures.
The interactive effects of food quality and temperature can be very important in understanding Daphnia distribution and production in lakes (Cole et al. 2002). Vertically migrating Daphnia can be expected to experience major fluctuations in both temperature and food quality over short time spans (Sterner and Schwalbach 2001). Especially, pond-dwelling species like D. magna may experience strong both seasonal and diurnal fluctuations in ambient temperature. For species subject to short-term fluctuations in temperature (and possibly also food quality), high temperatures facilitating high somatic growth rates (SGR) would pose the highest dietary demands for P (and N) in order to maximize SGR. Conversely, low temperatures would make the nutrient quota of food less relevant since SGR will be constrained by low temperature. Temperate herbivores commonly face strong variability in temperatures diurnally, annually and spatially. Zooplankton may face a fivefold temperature range from spring to summer and from surface to deep water layers, and their stoichiometric responses over this temperature range remain to be settled.
Our study is aimed towards the assessment of short-term responses in changing food quality and temperature. To test for the interaction of temperature and P limitation, we performed a factorial study where the SGR of the cladoceran Daphnia magna was assessed over a broad range of dietary C:P ratios (C:P = 125–790), and at different temperatures (10, 15, 20, and 25°C). This species commonly inhabits small water-bodies with dramatic diurnal and seasonal changes in temperature, and it has been shown previously that this species is sensitive to C:P ratios in its food (cf. Hessen et al. 2002).
There are two possible outcomes from these experiments. (1) The effect of dietary P limitation on SGR is greatest at low temperatures. This would be expected if the lower protein synthesis rates at low temperatures are compensated by a greater number of ribosomes, which entails more RNA, and, thus, an increased demand for P (Elser et al. 2000). (2) The effect of dietary P limitation is greatest at high temperatures were the SGR is highest.
Materials and methods
The green algae Selenastrum capricornutum Printz was grown at 20°C in chemostats at a dilution rate of 0.2 day−1 (20% of the volume continuously replaced over 24 h) on COMBO medium (Kilham et al. 1998) with the phosphate concentration reduced to 2 μM. The cultures reached steady states before experiment initiation. Details about the chemostats setup can be found in Hessen et al. (2002). We added several amounts of dissolved inorganic P (K2HPO4) to batches of the chemostat-grown, P-depleted, algae in order to “spike” them with P and lower their C:P ratio while keeping other food quality parameters constant (e.g., N, sterols, fatty acids). The P-depleted algae were incubated in darkness with the dissolved inorganic P for 30 min before we dispensed the algae into the new experiment vessels and onto filters for later measurements. About 1 h passed from when the inorganic P was added until the Daphnia were feeding on the spiked algae. The spiking procedure is based on the observation that P-depleted phytoplankton assimilate such inorganic P within a few minutes, yielding a shift in C:P (Rothhaupt 1995; Plath and Boersma 2001). We cannot completely rule out that there still were some changes in the macromolecular makeup of the algae during the course of the experiments, yet there are good reasons to assume that they should be minor compared with the direct P effects. The grazing experiments were performed in the absence of light to minimize macromolecular anabolism, and the chlorophyte used is practically devoid of key unsaturated fatty acids (cf. Leu et al. 2006). The spiking method is used as a published protocol designed for separating the effects of P and fatty acids (e.g., Rothhaupt 1995; Plath and Boersma 2001). See Table 1 for an overview of the C:P ratios of the algal diets. The algae were diluted to 3 mg C L−1 using N- and P-free COMBO medium before being used in the experiments. Algae culture C concentration for daily food dilution was calculated from the optical density (OD) at 633 nm using a previously established calibration curve between the OD and measured C concentration. Algae for C and P analysis were collected on pre-combusted (530°C, 3 h) GF/F filters (Whatman, Kent, UK). C contents were analyzed on a Thermo Finnigan FlashEA 1112 elemental analyzer. Samples for particulate P were analyzed using a modified molybdate blue method (Menzel and Corwin 1965) after persulphate digestion.
Table 2.
Summary statistics for the final model explaining Daphnia somatic growth rates
| Coefficients | Estimate | Standard error | t value | p |
|---|---|---|---|---|
| Intercept | −0.015 | 0.022 | −0.70 | 0.49 |
| Temperature | 0.018 | 1.2 × 10−3 | 15 | <2 × 10−16*** |
| C:P ratio | 5.6 × 10−5 | 4.8 × 10−5 | 1.2 | 0.25 |
| Experiment ID | −0.030 | 6.5 × 10−3 | −4.6 | 1.4 × 10−5*** |
| Temperature × C:P ratio | −1.1 × 10−5 | 2.6 × 10−6 | −4.3 | 3.4 × 10−5*** |
It explained 86% of the total variance, F statistics was 164.7 on 4 and 107 DF, the whole model p value was <2.2 × 10−16
*** Variable significance at the <0.001 level
The strain of Daphnia magna Straus used in all experiments had been kept in the laboratory for many generations at 20°C. In nature, D. magna are typically found in pond populations that during summer may experience strong temperature fluctuations. In shallow rock-pools typically inhabited by D. magna, temperatures at noon may approach 30°C on sunny days, and decrease to <15°C during night.
We conducted two factorial experiments, denoted as “A” and “B”, with either eight (A) or four (B) levels of algae P content (C:P = 125–790, mol:mol), and four temperatures (10, 15, 20, and 25°C). One of the food treatments was S. capricornutum grown in standard COMBO medium (50 μM inorganic P), the others were P-depleted algae with inorganic P added, which are referred to as “spiked” in order to separate the two types of algal food used. The C:P of these treatments [C:P = 125 (A) and 141 (B)] were close to those algae spiked to the lowest C:P ratios [C:P = 164 (A) and 187 (B)] (see Table 1). There were two replicates in experiment A, and three replicates in experiment B. Each replicate consisted of eight juveniles (from the second brood and <24 h old) transferred to a bottle with 50 mL of the treatment food. The dietary C:P ratios used ranged from less than the incipient limiting threshold for Daphnia (molar C:P 225–375 at 20°C; Brett et al. 2000) to ratios well above this threshold. Animals were moved to fresh food suspensions every 24 h. The experiments were run in darkness in temperature-controlled rooms.
Table 1.
The food types used in the experiments and the C:P-ratio variation within each type
| Experiment ID | Way of preparation | Average C:P | Standard deviation | n |
|---|---|---|---|---|
| A | Spiking | 792 | 183 | 5 |
| A | Spiking | 542 | 80 | 5 |
| A | Spiking | 471 | 109 | 5 |
| A | Spiking | 447 | 113 | 5 |
| A | Spiking | 383 | 95 | 5 |
| A | Spiking | 294 | 79 | 5 |
| A | Spiking | 187 | 73 | 5 |
| A | 50 μM P media | 141 | 42 | 5 |
| B | Spiking | 736 | 161 | 10 |
| B | Spiking | 487 | 85 | 10 |
| B | Spiking | 164 | 62 | 10 |
| B | 50 μM P media | 125 | 6 | 10 |
There were 5 replications (n) for each dietary treatment in experiment A (1 sample per day on five separate days), and 10 replications in experiment B (as in A but with 2 pseudoreplications each day)
Experiment A was terminated on day 6 and B on day 7, both before any eggs were deposited in brood pouches. B was carried out 1 day longer than experiment A in order to add one additional day of growth and to check if ontological development stage might disturb the growth rate pattern caused by diet and temperature. Within each experiment, the different temperature treatments where terminated on the same day.
The dry weights of individual D. magna were estimated using a regression relationship to pixel area on a digital image, according to Færøvig et al. (2002). SGR were calculated as [ln(w t) − ln(w 0)]/t, where w 0 is the dry weight at time zero and w t at time t. SGR were analyzed by linear models (lm-function in R) using temperature (continuous variable), C:P ratio in the food (continuous variable), food type (factor variable: spiked or not) and experiment ID (factor variable: A or B) as explanatory variables. A starting model with all four explanatory variables and their pairwise interactions was optimized by step-wise removal of the least important variable or variable interaction, as long as this removal improved the model performance. The models were evaluated using the Bayesian Information Criterion (BIC; Johnson and Omland 2004) and the model with the greatest support (lowest BIC) was selected as the best (Crawley 2007). In order to confirm the parameter selection based on BIC, we checked the significance of food type (spiked or not) and the parameters in the final model using likelihood ratio tests. We checked the residuals for indications of non-linearity, normality and heteroscedasticity during the model evaluation process, and the final model passed all the standard assumptions for linear regression. Statistics were carried out in R (R Development Core Team 2009).
Results
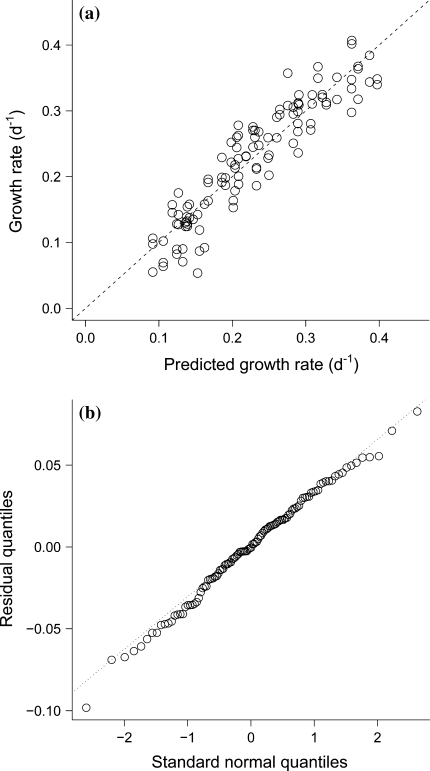
D. magna SGR varied from 0.1 to 0.4 day−1 and was affected by both experiment temperature and diet (Figs. 1, 2 and 3). The final model, which included temperature, food C:P ratio, experiment ID, and an interaction between temperature and food C:P, explained 86% of the variance in D. magna SGR and was highly significant (p < 2.2 × 10−16; see Table 2 for additional information). Observed versus predicted SGR (Fig. 1a) and a quantile–quantile plot (Fig. 1b) of the residuals confirm that the residuals were approximately normally distributed with constant variance. The slight non-linearity was mainly due to a single data point, and not considered worth pursuing. For each observation i, the final model is given by:
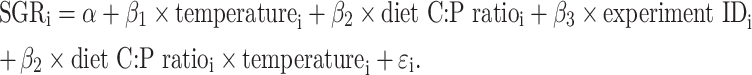 |
The parameters α and β are the population intercept and slope, unexplained information is captured by the residuals ε that are assumed to be normally distributed with an average of zero and a variance σ 2. Whether the food had been spiked with P or grown on a high P medium did not significantly affect the SGR and was not included as a factor in the final model (likelihood ratio test: F 1 = 0.36, p = 0.55). Both the diet × temperature interaction and experiment ID were higly significant (likelihood ratio tests: F 1 = 18.7 p = 3.4 × 10−5 and F 1 = 20.7 p = 1.4 × 10−5, respectively) and kept in the model.
Fig. 1.
a The somatic growth rate of Daphnia magna as predicted by the linear model plotted against the measured growth rates (r 2 = 0.86). The dashed line shows the 1:1 relationship. b Quantile–quantile plot
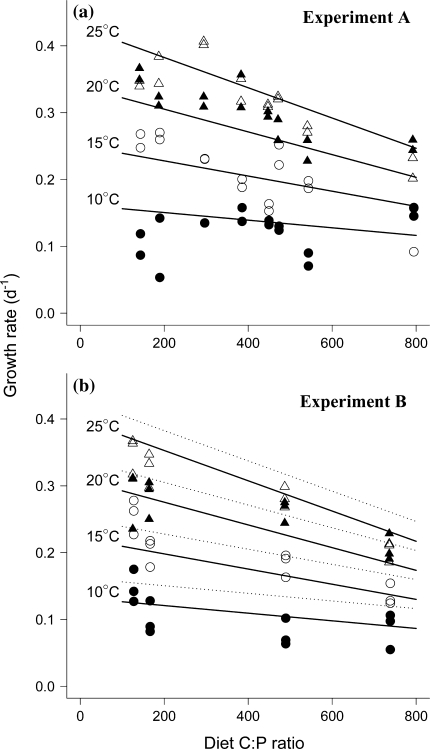
Fig. 2.
Daphnia magna somatic growth rates on diets of different food algae C:P at four temperatures: 25°C (open triangles), 20°C (solid triangles), 15°C (open circles), 10°C (solid circles). a Observations from experiment A, the solid lines display the model fit at each of the four temperatures. b Observations from experiment B. The solid lines display the model fitted to the data from experiment B, and the dotted lines show the model fitted to experiment A. For model statistics, see Table 2
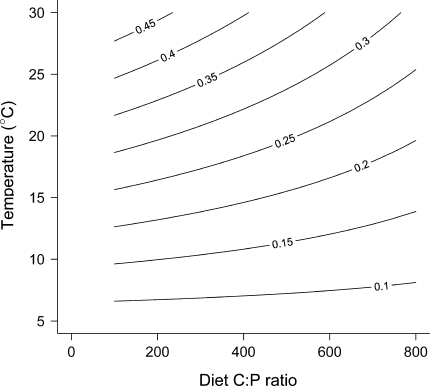
Fig. 3.
Contour plot of predicted SGR as function of temperature and diet C:P ratio (based on experiment a). Lines represent combinations of diet C:P ratio and temperature that are predicted to yield the same SGR (isoline labels; day−1)
D. magna SGR were dependent on both temperature and the diet C:P, with the highest SGR observed at high temperatures and low C:P ratios (Fig. 2; note that the lines in the figures are the model fit to the data and not regression lines). The SGR was still positive at the lowest temperature, but owing to the small size increments the results were rather scattered at 10°C. There was also a significant interaction between temperature and diet C:P ratios, such that diet C:P affected D. magna SGR less at low temperatures (Table 2, Fig. 2). There was a significant difference between the two experiments, but this effect was purely additive with no interaction with the treatment variables and represented only 2.7% of the total variance in SGR. Thus, while SGR from experiment B were lower than in A, the general response patterns were identical. Figure 3 illustrates this in a contour plot of predicted SGR as function of temperature and diet C:P ratio (experiment ID effect set to A). Mortality losses were negligible in both experiments.
Discussion
There were effects of both temperature and nutritional C:P-ratio on Daphnia SGR, confirming general expectations, yet the interaction between the two is quite intriguing and clearly shows that temperature reaction norms are influenced by food stoichiometry, and that the effect of dietary P limitation varies with temperature. The slope of the relationship between SGR and dietary C:P became less negative with decreasing temperature, to the extent that there was practically no effect of food stoichiometry on SGR at low temperatures. This finding implies that in situ temperature needs to be taken into account when assessing stoichiometric food quality effect on natural zooplankton populations.
Although the results of the two experiments were very similar, the significant interaction between them suggests that other factors than temperature and diet may slightly modify the dynamics of the animals’ growth. The instantaneous growth rate of developing Daphnia decrease with age, as reflected in RNA:DNA ratios (Gorokhova and Kyle 2002). The lower growth rates in experiment B are most likely an effect of this decreasing instantaneous growth rate as they grew for 1 day longer.
For temperate species like D. magna, our data suggest that while P deficiency can constrain SGR at most temperatures, enzyme kinetics or other aspects of food quality overrides the demands for P to RNA and protein synthesis at low temperatures. It has previously been demonstrated that growth and life history parameters in Daphnia generally did not respond to changes in temperature if food quantity was low, but did so when there were high food quantities (Orcutt and Porter 1984). This suggests that the temperature sensitivity of Daphnia may be similarly affected by reduced food quality as by reduced food quantity.
One possible concern is that food quality can affect the feeding rate of Daphnia (cf. Plath and Boersma 2001) and that the observed results could reflect that the available food supply was exhausted in the high C:P treatments, and thereby resulting in reduced growth due to food quantity limitation. However, previous studies using the same range of C:P, the same strain of Selenastrum and the same clone of D. magna have failed to demonstrate any significant effect on grazing rate owing to C:P (cf. Hessen et al. 2002; Darchambeau et al. 2003). It is, however, clear that grazing rate will be affected by temperature, and hence even if the grazing rate should be affected by C:P of the food, one could argue that this is part of the observed net response of grazer performance along the food quality and temperature gradients.
Dietary deficiency of essential polyunsaturated fatty acids is known to have greater impact on SGR at low temperatures (Masclaux et al. 2009), which is opposite to what we demonstrated here for P limitation. Since fatty acid and stoichiometric food quality most likely affect different mechanisms in organism SGR and reproduction, these effects could well operate independently, though with both being under the influence of temperature. We assumed that the P content was the only affecting food quality factor in our experiments; however, future work could ideally include a wider range of quality parameters to fully assess the relative importance of the various growth-promoting factors.
The elemental ratios in D. magna resemble those of other Daphnia species (Hessen 1990), which are generally known to show SGR retardation under P-deficient conditions (cf. Hessen et al. 2002). Hence, we believe the observed temperature-stoichiometry in D. magna could be relevant in a broader context, both on spatial and temporal scales within ecosystems. Herbivores could thus run the highest risk of P limitation during summer while elemental ratios in food are expected to be less relevant in colder seasons, or in habitats with low temperature such as the hypolimnia of lakes. Daphnia that migrate to the colder metalimnion (e.g., McLaren 1963; Zaret and Suffern 1976) might be less sensitive to dietary P limitation in this region, while at the same time the available food in the metalimnion is expected to be of higher P content due to higher nutrient content and lower light intensity at these depths. One prediction would be that temperate species inhabiting cold lakes should be less negatively affected by dietary C:P compared to those living at higher temperatures, and also that the increased temperatures observed (and expected) in lakes worldwide (cf. Adrian et al. 2009) would enhance the risk of dietary P limitation. We believe this could be relevant not only for Daphnia spp. but for P-limited heterotrophs in general, and would thus represent a climate-induced check on productivity of such species. Adding to this, elevated levels of CO2 may likewise induce elevated C:P in autotrophs, and thus increased risk of herbivore P limitation (Urabe et al. 2003). If herbivores additionally increase their requirement for dietary P in warmer temperatures, as the results from this study imply, the negative effects of food quality might get even more severe.
Acknowledgments
This project was financed by a grant in ecological stoichiometry from the Department of Biology, University of Oslo. J.P. was financed by the Swedish research council Formas. We also thank the editor and the reviewers for their help in improving this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Jonas Persson, Phone: +47-228-54577, FAX: +47-228-54001, Email: jonas.persson@bio.uio.no.
Marcin Włodzimierz Wojewodzic, Email: marcin.wojewodzic@bio.uio.no.
Dag Olav Hessen, Email: d.o.hessen@bio.uio.no.
Tom Andersen, Email: tom.andersen@bio.uio.no.
References
- Adrian R, O’Reilly CM, Zagarese H, Baines SB, Hessen DO, Keller W, Livingstone DM, Sommaruga R, Straile D, Van Donk E, Weyhenmeyer GA, Winder M. Lakes as sentinels of climate change. Limnol Oceanogr. 2009;54:2283–2297. doi: 10.4319/lo.2009.54.6_part_2.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett MT, Muller-Navarra DC, Park SK. Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton. Limnol Oceanogr. 2000;45:1564–1575. doi: 10.4319/lo.2000.45.7.1564. [DOI] [Google Scholar]
- Cole PC, Luecke C, Wurtsbaugh WA, Burkart G. Growth and survival of Daphnia in epilimnetic and metalimnetic water from oligotrophic lakes: the effects of food and temperature. Freshw Biol. 2002;47:2113–2122. doi: 10.1046/j.1365-2427.2002.00955.x. [DOI] [Google Scholar]
- Crawley MJ. The R book. Chichester: Wiley; 2007. [Google Scholar]
- Darchambeau F, Færøvig PJ, Hessen DO. How Daphnia copes with excess carbon in its food. Oecologia. 2003;136:336–346. doi: 10.1007/s00442-003-1283-7. [DOI] [PubMed] [Google Scholar]
- Elser JJ, O’Brien WJ, Dobberfuhl DR, Dowling TE. The evolution of ecosystem processes: growth rate and elemental stoichiometry of a key herbivore in temperate and arctic habitats. J Evol Biol. 2000;13:845–853. doi: 10.1046/j.1420-9101.2000.00215.x. [DOI] [Google Scholar]
- Færøvig PJ, Andersen T, Hessen DO. Image analysis of Daphnia populations: non-destructive determination of demography and biomass in cultures. Freshw Biol. 2002;47:1956–1962. doi: 10.1046/j.1365-2427.2002.00946.x. [DOI] [Google Scholar]
- Gorokhova E, Kyle M. Analysis of nucleic acids in Daphnia: development of methods and ontogenetic variations in RNA–DNA content. J Plankton Res. 2002;24:511–522. doi: 10.1093/plankt/24.5.511. [DOI] [Google Scholar]
- Hessen DO. Carbon, nitrogen and phosphorus status in Daphnia at varying food conditions. J Plankton Res. 1990;12:1239–1249. doi: 10.1093/plankt/12.6.1239. [DOI] [Google Scholar]
- Hessen DO, Anderson TR. Excess carbon in aquatic organisms and ecosystems: physiological, ecological, and evolutionary implications. Limnol Oceanogr. 2008;53:1686–1696. doi: 10.4319/lo.2008.53.4.1685. [DOI] [Google Scholar]
- Hessen DO, Færøvig PJ, Andersen T. Light, nutrients, and P:C ratios in algae: grazer performance related to food quality and quantity. Ecology. 2002;83:1886–1898. doi: 10.1890/0012-9658(2002)083[1886:LNAPCR]2.0.CO;2. [DOI] [Google Scholar]
- Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Evol Ecol. 2004;19:101–108. doi: 10.1016/j.tree.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Kerkhoff AJ, Enquist BJ, Elser JJ, Fagan WF. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob Ecol Biogeogr. 2005;14:585–598. doi: 10.1111/j.1466-822X.2005.00187.x. [DOI] [Google Scholar]
- Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia. 1998;377:147–159. doi: 10.1023/A:1003231628456. [DOI] [Google Scholar]
- Leu E, Færøvig PJ, Hessen DO. UV effects on stoichiometry and PUFAs of Selenastrum capricornutum and their consequences for the grazer Daphnia magna. Freshw Biol. 2006;51:2296–2308. doi: 10.1111/j.1365-2427.2006.01651.x. [DOI] [Google Scholar]
- Lovelock CE, Feller IC, Ball MC, Ellis J, Sorrell B. Testing the growth rate vs geochemical hypothesis for latitudinal variation in plant nutrients. Ecol Lett. 2007;10:154–163. doi: 10.1111/j.1461-0248.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- Masclaux H, Bec A, Kainz MJ, Desvilettes C, Jouve L, Bourdier G. Combined effects of food quality and temperature on somatic growth and reproduction of two freshwater cladocerans. Limnol Oceanogr. 2009;54:1323–1332. [Google Scholar]
- McLaren IA. Effects of temperature on growth of zooplankton and the adaptive value of vertical migration. J Fish Res Bd Can. 1963;20:685–727. [Google Scholar]
- Menzel DH, Corwin N. The measurement of total phosphorus in seawater based on the liberation of organically bound fractions by persulphate oxidation. Limnol Oceanogr. 1965;10:280–282. doi: 10.4319/lo.1965.10.2.0280. [DOI] [Google Scholar]
- Moore MV, Folt CL, Stemberger RS. Consequences of elevated temperatures for zooplankton assemblages in temperate lakes. Arch Hydrobiol. 1996;135:289–319. [Google Scholar]
- Orcutt JDJ, Porter KG. The synergistic effects of temperature and food concentration on life history parameters of Daphnia. Oecologia. 1984;63:300–306. doi: 10.1007/BF00390657. [DOI] [PubMed] [Google Scholar]
- Plath K, Boersma M. Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology. 2001;82:1260–1269. doi: 10.1890/0012-9658(2001)082[1260:MLOZSC]2.0.CO;2. [DOI] [Google Scholar]
- R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
- Reich PB, Oleksyn J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA. 2004;101:11001–11006. doi: 10.1073/pnas.0403588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhaupt KO. Algal nutrient limitation affects rotifer growth rate, but not ingestion rate. Limnol Oceanogr. 1995;40:1201–1208. doi: 10.4319/lo.1995.40.7.1201. [DOI] [Google Scholar]
- Sterner RW, Elser JJ. Ecological stoichiometry—the biology of elements from molecules to the biosphere. Princeton: Princeton University Press; 2002. [Google Scholar]
- Sterner RW, Schwalbach MS. Diel integration of food quality by Daphnia: luxury consumption by a freshwater planktonic herbivore. Limnol Oceanogr. 2001;46:410–416. doi: 10.4319/lo.2001.46.2.0410. [DOI] [Google Scholar]
- Urabe J, Togari J, Elser JJ. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Glob Chang Biol. 2003;9:818–825. doi: 10.1046/j.1365-2486.2003.00634.x. [DOI] [Google Scholar]
- Woods HA, Makino W, Cotner JB, Hobbie SE, Harrison JF, Acharya K, Elser JJ. Temperature and the chemical composition of poikilotherm organisms. Funct Ecol. 2003;17:237–245. doi: 10.1046/j.1365-2435.2003.00724.x. [DOI] [Google Scholar]
- Zaret TM, Suffern JS. Vertical migration in zooplankton as a predator avoidance mechanism. Limnol Oceanogr. 1976;21:804–813. doi: 10.4319/lo.1976.21.6.0804. [DOI] [Google Scholar]