Abstract
Objective:
To evaluate our learning-curve experience with laparoscopic management of endometrial carcinoma.
Methods:
Retrospective review of our first 125 patients with endometrial cancer who were managed laparoscopically. The patient population was reviewed in a chronological manner, noting patient demographics, operative procedure and times, estimated blood loss, hospital stay, complications, and pathology.
Results:
Overall, the mean age was 68.6 years (range 29-89), the mean weight was 160 pounds (range 97-328), and the mean Quetelet index was 27.8 (range 17.8-56.4). Metastatic disease was discovered in 28.8% (17/59) of patients with grade 2 or 3 lesions. There was no statistically significant variation in any of these parameters throughout the study. Operative times for staging without lymphadenectomy decreased significantly from a mean of 163 minutes to 99 minutes (p<.001). Operative times for staging with lymphadenectomy decreased from a mean of 196 minutes to 128 minutes (p<0.02). Hospital stay decreased from a mean of 3.2 days in the first quarter of our study to 1.8 days (p<.0001). The overall average complication rate of 4% (two enterotomies, two cystotomies, and a transected ureter) did not vary. However, the rate of conversion to laparotomy dropped significantly from 8% (2/25) to 0% (0/100).
Conclusions:
We found that operative times and hospital stays for laparoscopic staging of endometrial cancer continued to drop after 125 cases. While the ability to detect metastatic disease and the rate of major complications appear unrelated to length of the operator experience, the conversion rate to laparotomy decreases with operator experience. Learning-curve parameters must be recognized by physicians, patients, and researchers for a host of reasons.
INTRODUCTION
There are approximately 39,000 new cases of endometrial cancer diagnosed in the United States annually. Abdominal hysterectomy and adnexectomy have been the standard of care for many years. Lymphadenectomy has been routine for many of these patients since 1988, when the Federation of Gynecology and Obstetrics (FIGO) changed the staging of endometrial cancer from a clinical to a surgical approach.1 The new staging protocols required (a) assessment of the intraperitoneal cavity; (b) cytologic sampling; (c) adnexectomy; (d) lymphadenectomy; and (e) hysterectomy.
Recently, there have been many advancements in laparoscopic surgical techniques and instrumentation that have facilitated the transition of traditionally open surgical cases to operative laparoscopic operations. Laparoscopically assisted vaginal hysterectomy and complete hysterectomy along with laparoscopic pelvic and para-aortic lymphadenectomy have allowed operative laparoscopists to surgically stage patients with endometrial cancer.2–4
Not surprisingly, the use of laparoscopy in the surgical management of endometrial and other gynecologic malignancies is controversial. Proponents of operative laparoscopy espouse the adequacy, safety, and reduced recovery time. Skeptics of laparoscopy point out the problems of lymphadenectomy in obese patients and of the lack of randomized prospective studies. Traditionally, comparative randomized clinical trials have been the scientific gold standard to test new management approaches in oncology. Pioneers in laparoscopic surgery have been encouraged to institute randomized studies.5,6 Indeed, the Gynecologic Oncology Group (GOG) has initiated a randomized prospective study evaluating operative laparoscopic staging of patients with endometrial carcinoma.
Few studies to date have addressed at what point an operative laparoscopic surgeon has become accomplished enough to compare results of his laparoscopic technique to those of laparotomy. The laparoscopic learning-curve is often referred to but infrequently defined. While scant, the most data exist on general surgical procedures such as cholecystectomy and appendectomy. Publication of these data has been generated largely to address the issue of increased frequency of operative complications noted in neophytes. Even fewer reports exist on gynecologic surgery, and no data are currently available for gynecologic oncology procedures.
We review retrospectively our experience with our first 125 patients with endometrial cancer who were managed laparoscopically. Our purpose was to better define the learning-curve parameters such as operative time, blood loss, complications, hospital stay, and our ability to detect metastatic disease by this means.
MATERIALS AND METHODS
A retrospective review of the first 125 patients with laparoscopically managed preoperative clinical stage I endometrial cancer was carried out from May, 1991, through May, 1996. The charts were reviewed and the following data extracted: age, weight, Quetelet index, hospital stay, EBL, operative time, pathology, and operative complications. The results were evaluated by means with associated ranges. To evaluate the learning-curve, the data were evaluated over time for the 61 months of the study. The data were then subjected to statistical analysis using the Kruskall-Wallis Nonparametric ANOVA tests, Dunn's Multiple Comparison Test, and linear regression, with P values <0.05 being considered significant.
All operative procedures were performed by one or both of two authors (JMC and EAS). There was no selection bias such as age, weight, or previous surgeries. The operative procedure was done systematically. Initial evaluation of the peritoneal cavity was followed by the collection of peritoneal cytology. In those patients with preoperative grade 1 lesions, laparoscopic hysterectomy (either assisted or complete) was carried out and the uterus sent to pathology for frozen-section determination of both grade and depth of myometrial invasion. If the lesion was upgraded, or if the depth of invasion exceeded one-half the myometrial thickness, or if the tumor covered a majority of the anterior and posterior walls of the uterus, laparoscopic lymphadenectomy was performed. In patients with preoperative grade 2 or 3 lesions, the lymphadenectomy was performed prior to the hysterectomy. Lymph node sampling included pelvic nodes and/or para-aortic nodes.4 Operative times were obtained from the anesthesia record and EBL from the surgeon's estimation.
RESULTS
Overall, the patients had a mean age of 68.6 years (range 29-89), with a mean weight of 160.4 pounds (range 97-328). The mean Quetelet index is 27.8 (range 17.8-56.4). There was no statistical difference in patient age, weight, or bodymass index throughout the study.
Of the 125 patients, 65 underwent hysterectomy and adnexectomy, and 60 underwent hysterectomy, adnexectomy, and pelvic and/or para-aortic lymphadenectomy. Obesity precluded the para-aortic lymphadenectomy in three patients (Quetelet index = 47.2, 47.3, and 56.4).
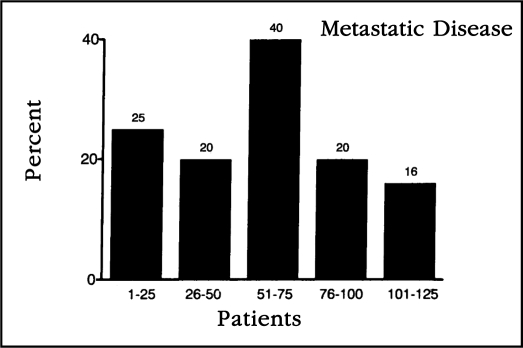
Overall, there were 66 grade 1, 34 grade 2, and 25 grade 3 malignancies. No metastatic disease was discovered in patients with well-differentiated tumors. Metastatic disease was discovered in 18% (6/34) of patients with grade 2 lesions and 44% (11/25) of patients with grade 3 lesions. There was no statistical difference (p= 0.29) throughout the study in our ability to surgically detect metastatic disease (Figure 1).
Figure 1.
This figure illustrates the percentage of patients in whom metastatic disease was discovered when plotted in chronological order in groups of 25 patients. No statistically significant difference was discovered in the percentage of metastatic disease discovered compared to operator experience.
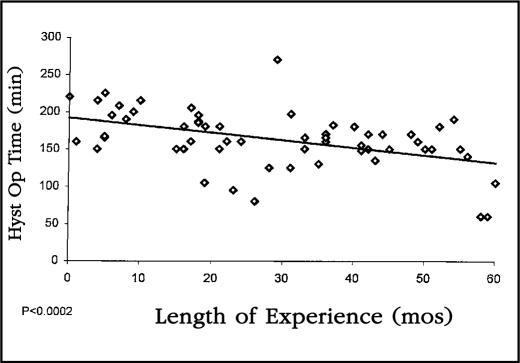
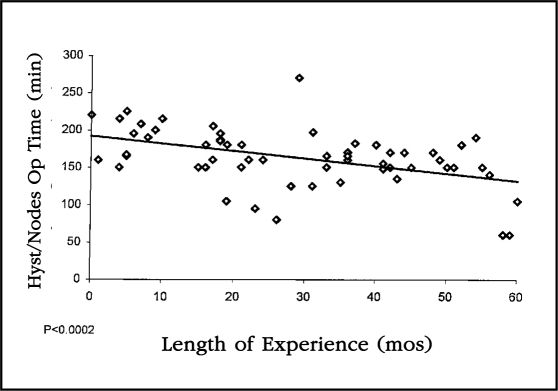
Operative times were noted separately between those patients undergoing combined hysterectomy and lymphadenectomy and those undergoing hysterectomy without lymphadenectomy. Operative times for staging without lymphadenectomy decreased from a mean of 163 minutes in the first quarter of our experience to 99 minutes in the final quarter. This decrease was highly significant statistically when plotted on the linear regression (p<0.0001) Figure 2. Similarly, the operative time for combined hysterectomy and lymphadenectomy decreased from a mean of 196 minutes in the first 25 patients to 128 minutes in the last 25. This decrease was also statistically significant when plotted on the linear regression (p<0.0002) (Figure 3).
Figure 2.
This linear regression curve plots the operative times for patients on whom laparoscopic staging was performed without lymphadenectomy. The decrease in operative time was highly statistically significant (p<0.0001).
Figure 3.
This linear regression curve demonstrates a highly statistically significant drop in operative time for patients who underwent laparoscopic staging with lymphadenectomy when compared to operator experience (p<0.0002).
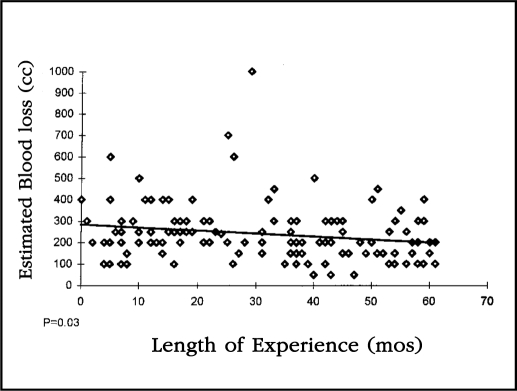
The mean estimated blood loss was 240 cc with a range of 50-1000. When the EBL of all patients was plotted on a linear regression curve, the drop was statistically significant (p= 0.03) (Figure 4).
Figure 4.
This linear regression curve looks at the estimated blood loss of all 125 patients in this study compared to length of operator experience. The slope of the curve indicates that the drop in blood loss was statistically significant (p=0.03).
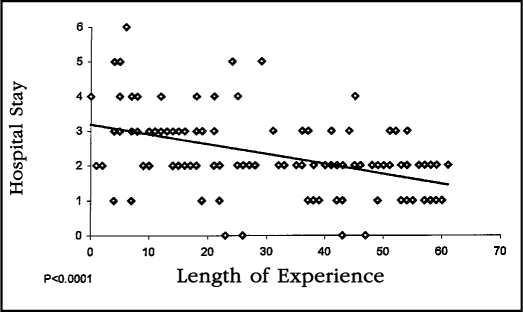
Length of hospital stay steadily decreased throughout the study period. The mean hospital stay for the initial 25 patients was 3.2 days (range 1-6 days) and decreased to a mean of 1.8 days (range 0-3) in the last 25 patients. The linear regression analysis of the hospital stay relative to operator experience was quite significant statistically (p<0.0001) (Figure 5).
Figure 5.
This linear regression curve of hospital stay compared with length of experience demonstrates a highly statistically significant decrease in hospital stay relative to operator experience (p<0.0001).
There were five significant operative complications: two cystotomies, one colotomy, one enterotomy, and one ureteral injury. All but the latter were recognized intraoperatively. Two injuries occurred in the first 25 patients, one in the third group of 25, and two in the fourth group of 25 patients in our study. The first two injuries required laparotomy for repair. The last three injuries (an enterotomy during adhesiolysis, a transverse colotomy from primary trocar insertion, and a cystotomy from a suprapubic trocar) were all repaired laparoscopically. There was no appreciable difference in the rate of major complications throughout the study period. However, the laparotomy rate dropped impressively, from 8% (2/25) in the first 25 patients to 0% (0/100) in the final 100 patients of the study.
DISCUSSION
Very little is known about the operative laparoscopic learning- curve in any surgical specialty. Early evaluation of laparoscopic cholecystectomy has demonstrated that competency (defined by one's ability to perform the intended procedure with complication rates similar to those of traditional surgery) can be attained, but that a long learning curve, requiring as many as 300 cases, may be needed to acquire adequate experience.7–11 Similarly, the learning curve appears to be long for gynecologic procedures. Weber et al demonstrated a continued drop in the time required to perform ovarian cystectomy in their experience of more than four years.12 Likewise, Harkki-Siren et al demonstrated a continuous drop in the operative time required to perform their first 100 laparoscopic hysterectomies. 13 No data exist on the learning experience in gynecologic malignancies.
Data on the learning experience are important for a number of reasons. Individual physicians need to be aware of the number of procedures and the length of time required to master a new laparoscopic technique. Patients should be cognizant of this phenomenon and aware of their surgeon's experience. Interpretation of current literature must take into account the learning hurdle. Randomized trials, designed to make fair comparisons, must take the learning curve into consideration when comparing laparoscopy to laparotomy. When can an accomplished laparotomist compare his laparoscopy and laparotomy skills? Our data show that statistically significant reductions in operative time occur and continue to occur after 125 patients and five years of time. The current GOG protocol comparing endoscopic and open surgical staging requires that participating oncologists (experienced laparotomists) have taken a laparoscopy course and have performed two adequate laparoscopic pelvic and para-aortic lymphadenectomies.
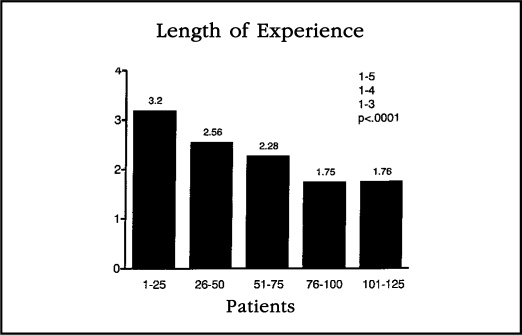
Our data demonstrated the reduction in length of hospital stay was highly significant with regard to operator experience (Figure 5). When the data are examined in groups of 25 patients, the length of stay appears to have leveled off after 75 patients to around 1.75 days (Figure 6). We believe this hospital stay learning-curve reflects the learning of surgeons as well as hospital nurses. Surgeons have improved their skills, and both surgeons and nurses have learned that patients can go home earlier than before. We routinely educate our patients preoperatively concerning expectations about a short hospital stay and recovery time. It should be noted that hospital stays for laparotomy have decreased in most institutions over this time as well. However, we do not believe that hospital stays for staging of patients via laparotomy will approach the 1.75 day range.
Figure 6.
This bar graph represents the hospital stay of patients in chronological order when plotted in groups of 25. There appears to be a leveling off of hospital stay after the first 75-100 patients.
The detection of metastatic disease appears to be independent of operator experience. This would suggest that the procedure itself is adequate from the beginning. Our overall rate of metastatic disease of 29% (17/59) for grade 2 and 3 lesions is comparable to that of surgical staging operations performed via traditional laparotomy.14–16
Our rate of major complications is comparable to that of recently reported studies in surgical staging via laparotomy. These reports indicate that thromboembolic events occurred in 1.2-9.5%, transfusion rates of 6-10%, fistulas 0-2%, myocardial infarction 0-5.7% , evisceration 0-1% , ureteral injury 0-1.2%, and mortality 0-0.7%.17–19 We had no thromboembolic events, had a 2% transfusion rate, and two of the major complications we encountered were small cystotomies. This lack of association of complications with operator experience has been demonstrated by other authors.13
Since many patients with endometrial cancer are obese, some physicians are concerned about their ability to perform laparoscopic surgery in the majority of patients who have been evaluated preoperatively. Kadar et al recently published their experience with laparoscopic lymphadenectomy in ten patients weighing at least 180 pounds (mean 212 pounds).20 Their ability to successfully accomplish retroperitoneal surgery was echoed by our own experience, in which we were able to stage 22 patients laparoscopically who weighed >180 pounds (mean 205.5). Only three patients had incomplete staging directly related to obesity (Quetelet index 47.3, 47.2, and 56.4 respectively). The well-known association of well-differentiated endometrial carcinomas and obesity works to the laparoscopist's advantage if lymphadenectomy is avoided in superficially invasive grade 1 lesions.
Our results should be interpreted with the knowledge that our surgeons performed concomitantly more than 700 additional operative laparoscopic procedures for benign and malignant disease during the study period. These learning curves reflect the experience of surgeons dedicated to operative laparoscopy, and we believe the rate of operative laparoscopic experience is one important parameter of any individual learning curve.
Our results indicate that physicians trained in laparoscopic lymphadenectomy can safely offer laparoscopic staging to their patients. As neophytes, their procedures probably will be longer and their patients probably will remain hospitalized longer than those of their more experienced colleagues, but the adequacy and safety of their initial procedures probably will equal those of experienced laparoscopists. Prospective randomized trials comparing laparoscopy with laparotomy should not compare operative times, hospital stays, conversions to laparotomy, or estimated blood loss unless the surgeons have adequate laparoscopic experience.
References:
- 1. FIGO Stages: 1988 revisions. Gynecol Oncol. 1989; 35: 125–127 [Google Scholar]
- 2. Childers JM, Hatch K, Surwit EA. The role of laparoscopic lymphadenectomy in the management of cervical carcinoma. Gynecol Oncol. 1992; 47: 38–43 [DOI] [PubMed] [Google Scholar]
- 3. Querleu D. Laparoscopic para-aortic lymph node sampling in gynecologic oncology: A preliminary experience. Gynecol Oncol. 1993; 49: 24–29 [DOI] [PubMed] [Google Scholar]
- 4. Childers JM, Brzechffa PR, Hatch KD, Surwit EA. Laparoscopic-assisted surgical staging (LASS) of endometrial cancer. Gynecol Oncol. 1993; 51: 33–38 [DOI] [PubMed] [Google Scholar]
- 5. Pitkin RM. Operative laparoscopy: Surgical advance or technical gimmick? Obstet Gynecol. 1992; 79: 441–442 [DOI] [PubMed] [Google Scholar]
- 6. Grimes DA. Frontier of operative laparoscopy: A review and critique of evidence. Am J Obstet Gynecol. 1992; 166: 1062–1071 [DOI] [PubMed] [Google Scholar]
- 7. The Southern Surgeons Club: A prospective analysis of 1,518 laparoscopic cholecystectomies. N Engl J Med. 1991; 324: 1073–1078 [DOI] [PubMed] [Google Scholar]
- 8. Peters JH, Ellison C, Innes JT., et al. Safety and efficacy of laparoscopic cholecystectomy: A prospective analysis of 100 initial patients. Ann Surg. 1991; 213: 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sariego J, Spitzer L, Matsumoto T. The learning curve in performance of laparoscopic cholecystectomy. Int Surg. 1993; 78: 1–3 [PubMed] [Google Scholar]
- 10. Kagir B, Rangraj M, Maffuci L, Herz BL. The learning-curve for laparoscopic cholecystectomy. J Lap Surg. 1994; 4: 419–427 [DOI] [PubMed] [Google Scholar]
- 11. Dashow L, Freidman I, Kempner R, Rudick J, McSherry C. Initial experience with laparoscopic cholecystectomy in the Beth Israel Medical Center. Surg Gen Obstet. 1992; 175: 25–30 [PubMed] [Google Scholar]
- 12. Weber BM, Long CA, Cowan BD. Laparoscopically directed ovarian cystectomy in premenopausal women: Impact of surgical experience on surgical time. J Reprod Med. 1995; 40: 273–276 [PubMed] [Google Scholar]
- 13. Harkki-Siren P, Sjoberg J. Evaluation and the learning curve of the first 100 laparoscopic hysterectomies. Acta Obstet Gynecol Scand. 1995; 74: 638–641 [DOI] [PubMed] [Google Scholar]
- 14. Creasman WT, Boronow RC, Morrow CP, DiSaia PJ, Blessing JA. Adenocarcinoma of the endometrium: Its metastatic lymph node potential. Gynecol Oncol. 1976; 4: 239–244 [DOI] [PubMed] [Google Scholar]
- 15. Boronow RC, Morrow CP, Creasman WT, DiSaia PJ. Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984; 63: 825–832 [PubMed] [Google Scholar]
- 16. DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrence patterns in stage I endometrial carcinoma. Am J Obstet Gynecol. 1985; 151: 1009–1015 [DOI] [PubMed] [Google Scholar]
- 17. Orr JW, Holloway RW, Orr PF, Holiman JL. Surgical staging of uterine cancer: An analysis of perioperative morbidity. Gynecol Oncol. 1991; 42: 209–216 [DOI] [PubMed] [Google Scholar]
- 18. Homesley HD, Kadar N, Barett RJ, Lentz FS. Selective pelvic and para-aortic lymphadenectomy does not increase morbidity in surgical staging of endometrial cancer. Am J Obstet Gynecol. 1992; 167: 1225–1230 [DOI] [PubMed] [Google Scholar]
- 19. Larson JM, Johnson K, Olson KA. Pelvic and para-aortic lymphadenectomy for surgical staging of endometrial cancer: Morbidity and mortality. Obstet Gynecol. 1992; 79: 998–1001 [PubMed] [Google Scholar]
- 20. Kadar N. Laparoscopic pelvic lymphadenectomy in obese women with gynecologic malignancies. J AAGL. 1995; 2: 81–85 [DOI] [PubMed] [Google Scholar]