Abstract
Objective:
To assess the efficacy and safety of Insuflow® (Georgia BioMedical, Inc.) filter heater hydrator device in reducing the incidence, severity and extent of hypothermia, length of recovery room stay and postoperative pain at the time of laparoscopy.
Design:
Prospective, randomized, blinded, controlled multi-center study. Patients underwent gynecologic procedures via laparoscopy; surgeons, anesthesiologists and recovery room personnel assessed the results.
Setting:
Seven North American institutions.
Patients:
Seventy-two women for safety evaluation and efficacy studies.
Interventions:
Intraoperative pre-conditioning of laparoscopic gas with the Insuflow® device (treatment) or standard raw gas (control) during laparoscopic surgery and postoperatively.
Main Outcome Measures:
Incidence, severity and extent of hypothermia, postoperative pain perception and length of recovery room stay.
Results:
The Insuflow® group had significantly less intra-operative hypothermia, reduced length of recovery room stay and reduced postoperative pain. Pre-conditioning of laparoscopic gas by filtering heating and hydrating was well tolerated with no adverse effects. The safety profile of the Insuflow® pre-conditioned gas showed significant benefits compared to currently used raw gas.
Conclusions:
Pre-conditioning laparoscopic gas by filtering heating and hydrating with the Insuflow® device was significantly more effective than the currently used standard raw gas and was safe in reducing or eliminating laparoscopic-induced hypothermia, shortening recovery room length of stay and reducing postoperative pain.
Keywords: Gas hydration, Laparoscopic hypothermia, Pain, Length of stay, Laparoscopy, Peritoneum
INTRODUCTION
Changes induced by the currently used raw gas during insufflation to create a pneumoperitoneum induces laparoscopic hypothermia, causes postoperative pain and results in prolonged recovery room stay.1–7 Because the gas must be bone dry, there is a stark difference between the characteristics of this regulated raw gas and the normal physiologic condition of the abdomen that causes this dramatic contrast. The difference between the temperature of standard raw gas of 21 degrees Centigrade (C) and 37.0 degrees C core temperature and no water vapor versus intra-abdominal steady state satu-ration results in alterations that upset normal abdominal homeostasis. These changes are iatrogenically induced due to these differences and the insufflation gas delivery system. The result is alterations that influence development of hypothermia, effect recovery room length of stay and postoperative pain perception. The annual cost of correcting for the iatrogenic intra- and postoperative con-sequences of laparoscopy due to prolonged recovery room stay and productive work loss in the United States is estimated to be between $2.26-1.56 billion per year. Changes that improve laparoscopic gas characteristics from its raw state to a more physiologic condition influences surgical outcome, reduces pain, has cost benefit and improves the level of safety. This recognition is compelling and establishes a new standard of care.
Principles of any surgery including laparoscopy include gentle tissue handling, reduction of foreign body contamination and prevention of tissue drying. The use of standard unconditioned gas is a contradiction to these long-standing principles. In the current raw state the gas and its delivery system contribute to foreign body contamination and tissue dessication. Maintaining or reproducing the normal physiologic intra-abdominal environment (contaminant free, warm and moist) is a standard that can be achieved and should be met.
Laparoscopic gas filters were introduced in 1989.1 It is long understood that the laparoscopic gas is a contributor to surgical hypothermia.5 Attempts to correct laparoscopic hypothermia have been found inadequate by only heating the gas. It is known that without hydration only heating the gas has little effect on preventing laparoscopic hypothermia.2,3 It is necessary to use heated gas containing water vapor to have maximal intraoperative heat preservation and be appropriate for tissue surfaces to result in minimizing tissue damage. The detrimental changes that occur due to the raw gas require modification by heating and hydrating.2,3 A method to deliver pre-conditioned gas that accomplishes these necessary changes that modify standard raw gas to one more appropriate for laparoscopic procedures is advocated.2,3 The use of heated gas compared to the current raw gas is found to decrease postoperative pain.6–8 Methods that only heat laparoscopic gas in the insufflator or in warmed gas tubing are only marginal in their ability to heat, transmit and maintain the gas at a physiologic temperature when it enters the abdomen.6 To the best of our knowledge, only one report describes the use of heated humidified gas which was shown to reduce the time to return to normal function and decrease postoperative pain.7 A recently approved method and device is available that filters, heats and hydrates laparoscopic gas to a more physiologic condition as it enters the peritoneal cavity. The purpose of this study is to assess the efficacy of this device (Insuflow®), a method that pre-conditions by filtering heating and hydrating endoscopic gas, and to determine its effect in reducing laparoscopic-induced hypothermia, reducing pain and shortening recovery room length of stay.
MATERIALS AND METHODS
Study Design
This prospective, randomized, blinded, controlled, multi-center study compared the efficacy and safety of the Insuflow device regarding the incidence, extent and severity of laparoscopic-induced hypothermia, postoperative pain perception and effect on the length of recovery room stay.
Randomization
Before the procedure, patients at each center were randomly assigned to the Insuflow® (treatment group) or the raw gas (control group).
The Insuflow system has three components: an AC/DC converter, a controller circuit and a disposable filter heater hydrator (Insuflow®) device. The converter delivers reliable safe electric current and is connected to a controller circuit box that adapts and retrofits to any insufflator. The Insuflow device attaches to the controller circuit box and insufflator. It provides filtration by a hydrophobic 0.2 micron CO2 Guard® filter and is nine feet long. The heating and hydrating occurs within five centimeters of the intra-abdominal gas delivery point for delivery of optimally conditioned gas. The small heater hydrator section is filled with eight cubic centimeters of warm sterile water, normal saline or lactated ringers solution for each 150 liters of gas insufflation used during the procedure. The insufflator settings are independent of and not effected by the Insuflow® device. The gas is delivered to the patient at 36.2° C (97.2° F) and 95% relative humidity at constant low flow demand for 150 liters of gas (2 hours 30 minutes average). The intra-abdominal delivered gas characteristics can vary due to the demands placed on the device by the user and are dependent on gas flow rate, frequency and amount of gas evacuated.
Test Conditions
All centers used their own insufflators. All surgeons determined their own parameter settings for gas insufflation (flow rates and intra-abdominal pressure), followed their own institutional and personal standards for surgical procedures, set their own operating room temperatures, irrigation use and gas evacuation criteria. All anesthesiologists followed and determined anesthetic characteristics and individual methodologies for the surgical procedure. Individual institutional recovery room protocols were followed. No surface warming devices were used in any of the Insuflow® patients. Operating room ambient temperature and humidity were recorded and not modified during the procedures.
Study Population
Eight principal investigators at seven institutions enrolled 72 adult women in the Insuflow® and control groups from January 15, 1998 to May 29, 1998. The patients were adult women between 18 and 48 years of age. Patients' weights were between 97 and 252 pounds. All patients had laparoscopy. The protocol excluded pregnant or cancer patients.
Surgery
The methodology and care of all patients was consistent with the investigators' standard surgical and medical practices. The methodology and care of patients in the two groups differed only in the use of the Insuflow® device for the study group. Data was kept of various characteristics of the procedure, insufflation and medication use.
Evaluation
Preoperative evaluation included medical and surgical history, vital signs (temperature, blood pressure and heart rate), laboratory values (complete blood count and pregnancy test), eligibility criteria and informed consent documentation.
Intraoperative evaluation was done following each institution and physician's established practices. Evaluation of the following parameters was done every ten minutes during and after surgery including operating room temperature and humidity, patient core temperature monitoring by endotracheal temperature probe, insufflation gas volume, flow rate and pressure and irrigation volume consumed. Pain questionnaires were used with a visual analogue scale having scores from 0 (no pain) to 10 (unbearable pain) using established protocols.9
Post-anesthesia recovery room evaluation followed each institution's established protocols and included temperature, pain medication use, time in recovery room and pain perception.
Evaluation of Safety
Throughout the study no adverse events occurred in the Insuflow® group. Physician and patient evaluations were solicited and evaluated.
Statistical Analysis
All variables were summarized with descriptive statistics, including number, mean, median, standard deviation, range of continuous variables and number and percentage in each category.
RESULTS
There were no statistically significant differences between the groups in demographic information. Surgical evaluations included diagnoses of uterine leiomyomata, infertility, pelvic adhesions and endometriosis. A total of 72 patients were in the study at seven different centers.
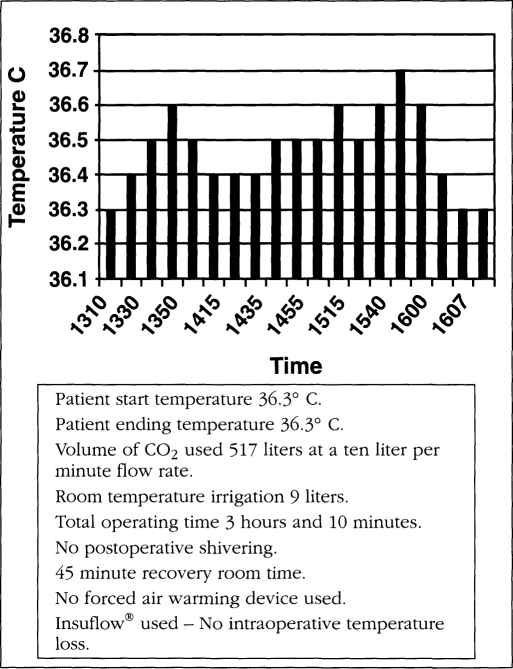
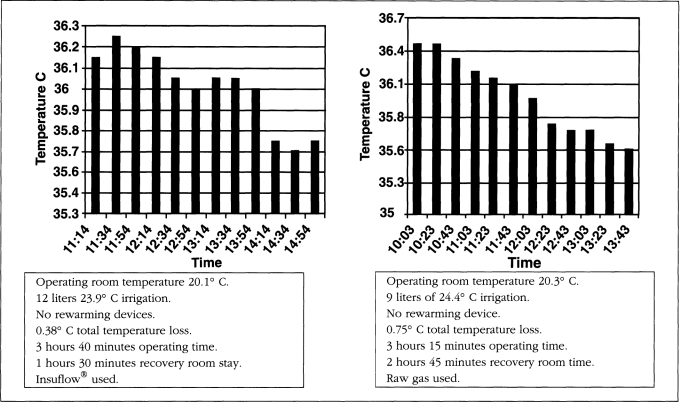
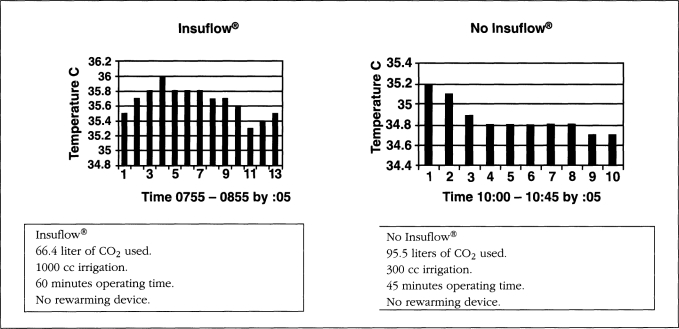
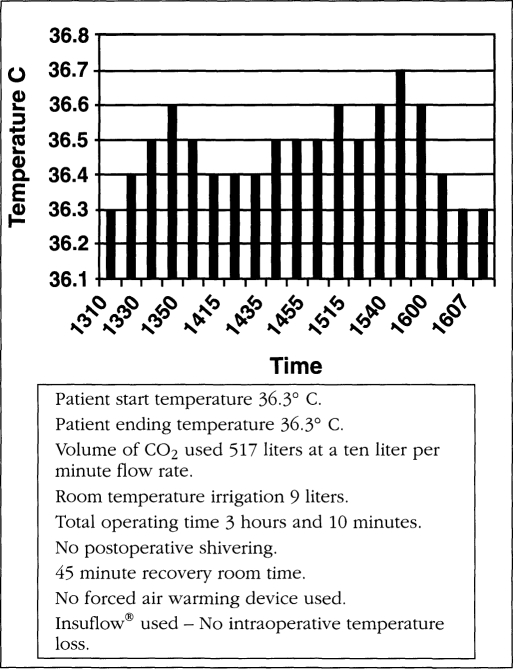
All patients started their procedures in a euthermic state. Operating room temperature ranged from 19.5-21.5° C (67.1-70.7° F). Relative humidity ranged from 42-59%. Operating time ranged from 38 to 262 minutes. The total intraoperative temperature drop in the Insuflow group ranged from 0.0-0.6° C (average total procedure loss 0.3° C, less than 0.1° C per hour) and 0.3-2.06° C (average total loss per procedure was 1.64° C, more than 0.6° C per hour) for the raw gas group. Carbon dioxide gas volume ranged from 82-680 liters. Irrigation volume ranged from 0.3-12 liters and at time of use was 26° C (78.8° F) or less. All cases utilized laser or electro-surgical devices. Figures 1–3 depict findings for three procedures in both groups. While procedures less than 35 minutes had little or no hypothermia, pain profiles for these short procedures were still statistically significantly improved in the Insuflow® group.
Figure 1a.
No Insuflow®.
Figure 2.
Insuflow® versus raw gas comparison.
Figure 3.
Body Temperature.
Figure 1b.
Insuflow® device used.
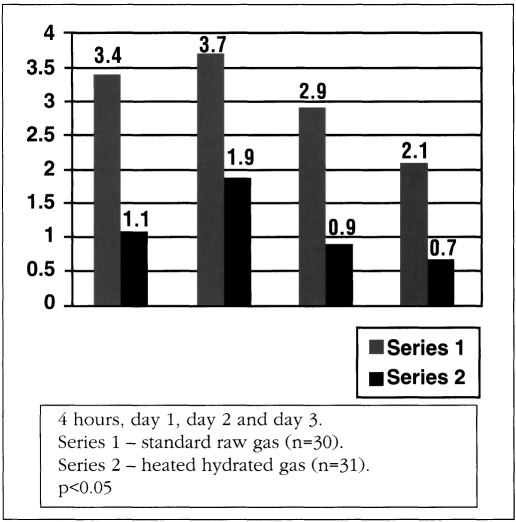
A total of 88.9% of questionnaires were completed. A significant difference in postoperative pain was found in the raw gas versus the Insuflow® warmed hydrated gas group. Consistently lower pain values were found in the Insuflow® group at all time intervals up to three days. Pain intensity was directly related to gas volume used and length of surgery in both groups but was statistically significant and reduced in the Insuflow® group. Shoulder and sub-phrenic pain were significantly reduced in the Insuflow® group at all time parameters compared to the standard raw gas group regardless of volume of gas consumed or length of surgery (Figure 4).
Figure 4.
Shoulder pain.
Efficacy
The Insuflow® group had a statistically significant reduction in intraoperative and postoperative hypothermia for procedures lasting over one hour, reduced pain perception postoperatively for all procedures regardless of length of surgery or gas consumed and shorter recovery room stay compared to the standard raw gas group. There was no reduction or enhancement effect of laser or electrosurgery with the Insuflow device.
Safety
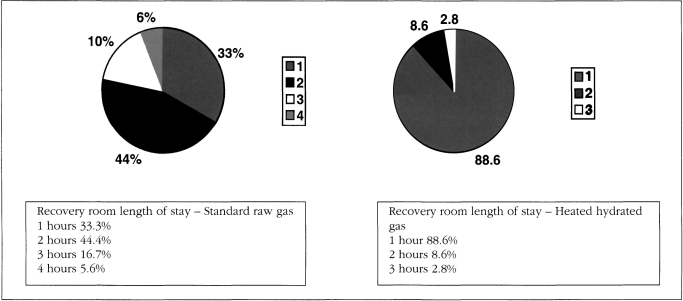
The safety profile of the Insuflow® device showed an improvement over the currently used raw gas group. No adverse effects were noted intraoperatively or postoperatively in the Insuflow group. Statistically significant differences were noted between the groups regarding hypothermia, pain perception and recovery room length of stay. These parameters showed that the Insuflow® group had significantly less intraoperative and postoperative hypothermia (8.3% versus 94.4%), postoperative pain improvement (56%) and shorter recovery room length of stay (88.9% versus 33.3% less than one hour). No Insuflow® patient had a recovery room stay longer than two hours. No Insuflow® patients became more than mildly hypothermic (36.0° C or 96.8° F). In the raw gas group, 44.4% were in the recovery room for two hours, 16.7% for three hours and 5.6% over four hours (Figure 5). For the raw gas group, 100% of patients were mildly hypothermic if the procedure was longer than 1 hour and 30 minutes, or if more than 110 liters of gas were used.
Figure 5.
Comparison of length of stay standard raw gas versus heated hydra ted pre-conditioned gas.
DISCUSSION
Caeteris parabus, insufflation with standard current raw gas into the peritoneal cavity, results in physiologic changes directly attributable to the nature of the gas, ie, tissue dessication, hypothermia and postoperative pain.
The gas standard as promulgated by the United States Pharmacopeia and National Formulary defines gas characteristics for purity and requires less than 200 parts per million of water vapor (bone dry).10 Surgical principles and techniques that influence laparoscopic outcome include attention to detail, gentle minimal tissue handling, meticulous hemostasis, reduction of foreign body contamination, prevention of tissue drying or dessication and magnification when appropriate.11,12 Therefore, it is important and beneficial to maintain or reproduce the normal physiologic environment of the abdomen during any surgery to ensure optimal outcome, including laparoscopy. This is not the case using the current raw gas. The gas delivery systems further compound the problem by throttling gas to extremely high flow rates. The normal abdominal environment is particle free, 37° C and has tissue surfaces moist with peritoneal fluid. The current standard raw gas (usually carbon dioxide) and delivery systems used to create a laparoscopic pneumoperitoneum contain contaminants (inorganic and organic debris from cylinders and insufflators), is 21° C (69.8° F) and is bone dry (0.0002% water vapor).1,13,14
The steady state of high water vapor content in the abdomen with the peritoneal fluid covering tissue sur-faces is severely altered by insufflation of the standard raw bone dry gas. Insufflation with CO2 in rigorous experimental studies demonstrated significant fall in core temperature.2 Experiments further demonstrate these differences by measuring and comparing the temperature and relative humidity differences for gas insufflation using cold dry gas versus warmed humidified gas to be 0.6° C per hour.3 Prevention of water loss is the most important factor in preventing laparoscopic hypothermia. The difference between the warm moist tissue surfaces and the cool dry gas causes rapid evaporation resulting in tissue dessication, mesothelial damage and loss of peritoneal cells with exposure of the underlying connective tissue matrix. Immediately after peritoneal drying there is loss of mesothelial cells on the cecum.15
The laparoscopic gas delivery system throttles gas through small apertures at high flow rates resulting in a nozzle effect and jet streaming of the gas. The fluid dynamics of the gas delivered through constricted apertures at high flow rates on wet tissue surface leads to rapid evaporation, heat loss resulting in hypothermia, tissue dessication and peritoneal damage. This is the case when gas is delivered through a Veress needle, small diameter trocars or through ports that have a similar-sized object entering through a port (10 mm laparoscope through a 10 mm trocar). The result is extremely high flow jet streams of cold dry gas over wet tissue surfaces, severe local tissue hypothermia and generalized evaporative hypothermic effects.16 This creates a condition akin to a wind chill effect from the nozzling of gas through small diameter openings onto the peritoneal sur-faces. Larger, unobstructed gas entry ports with low gas flow rates reduce deleterious effects on tissue from gas insufflation.
Factors that surgeons have control over that contribute to these problems are gas flow rate demanded, insufflator settings, amount of gas consumed, length of surgical exposure, gentleness of tissue handling, operating room temperature and temperature of irrigation solutions. Factors that contribute to operative hypothermia over which there is no control is patient age, size, pre-existing metabolic conditions and prior medication use. Awareness by anesthesiologists of operative hypothermia has long been noted. Anesthesiologists recognized the need for pre-conditioning gas delivered to the respiratory tract in the 1950's. It was determined that adding water vapor to gases prevented damage to the trachea and lung tissue. Heating and humidification devices that prevent tissue drying and hypothermia effects from respiratory tract gas delivery have been standards of care for over 35 years. The most recent effort to help control intraoperative laparoscopic hypothermia is the addition of surface convection warming devices. While any and all attempts to reduce hypothermia are desirable, this is an attempt to cure a problem after it has occurred. The prevention of a deleterious iatrogenic effect before it occurs is a more prudent approach. Pre-conditioning gas with heat and water vapor has been advocated2,3 and is a practical technologic cost-effective reality with the Insuflow® device. The device is efficient, rapidly responds to use demands of insufflation and maintains a more normal intra-abdominal condition compared to using raw gas.
Intraoperative hypothermia requires extensive monitoring, care and time in the post-anesthesia recovery room. Postoperative hypothermia has both metabolic and economic costs. Normal core body temperature is 37° C. Clinical hypothermia is defined as a core temperature less than 36° C (96.8° F).17 Changes resulting from mild and moderate hypothermia (32-35° C) are significant. Surgery stresses normal body temperature, normal regulatory mechanisms are modified and the balance of heat production and heat loss is altered. The result is a shift of temperature outside the normal physiologic range. The thermoregulatory system attempts to maintain the core temperature in a 0.2° C range. Intraoperative maintenance of the preoperative normothermic state is necessary to counteract the negative effects of hypothermia as they occur during surgery. Even mild operative hypothermia influences skin infection rate and perioperative wound infection by directly impairing immune function and vasoconstriction (by decreasing partial pres-sure of oxygen in tissues), induces hypokalemia, impairs myocardial function, depresses respiration, influences nitrogen balance,18 depletes clotting factors, induces thrombocytopenia,19 decreases activation of the coagulation cascade,19 decreases collagen synthesis,20 impairs chemotaxis and phagocytosis of neutrophils and decreases the production of antibodies. Mild hypothermia alters drug metabolism and pharmacokinetics, significantly prolongs recovery room time21,22 and is associated with postoperative shivering, substantial adrenergic activation23 and patient discomfort.24 A core temperature decrease of 1.5° C causes a three times increase in ventricular tachycardia,25 increased postoperative ventilation, oxygen consumption and changes peripheral vascular tone.
The normal thermal steady state is one in which heat loss is equal to metabolic heat production. This is not uniformly distributed during surgery. Thermoregulatory mechanisms try to keep the core temperature nearly constant while the periphery is simultaneously at a lower temperature because of tonic vasoconstriction.26 Due to the evaporative cooling effects caused by the dry laparoscopic gas, intra-abdominal conditions are altered, thus decreasing the heat available for redistribution. This upsets the normal balance of heat retention and loss. The heat normally sequestered in the abdomen is lost by vaporization of water from peritoneal surfaces, gas removal and as yet undetermined reflexes that contribute to thermal instability. This normal core heat sink is then unavailable for postoperative thermoregulation and redistribution and leads to a cool periphery and a cool core compounding the hypothermic effect. The use of heated laparoscopic gas containing water vapor allows for maintenance of the normal heat sink and reduction or prevention of the induced hypothermia.
The alterations caused by peritoneal dessication result in intact mesothelial cells becoming absent from bowel sur-faces immediately after drying.15 The effect of damaged peritoneum is denuded areas with release of chemically active kinins and prostaglandins that contribute to post-operative pain. The damaged peritoneum increases the susceptibility to adhesion formation from apposing defects and probably contributes to de-novo adhesion formation. The application of heated gas containing water vapor during insufflation allows for maintenance of the normal intra-abdominal condition to maximize peritoneal preservation at laparoscopy.
Postoperative pain intensity has been shown to decrease when heated laparoscopic gas is utilized.6 Heated humidified gas has been shown to improve return to normal activities by 54% in cholecystecomy procedures and significantly reduce pain extending to the third postoperative day.7 The conclusions of these studies are that warmed CO2 gas leads to significant reduction of pain, and humidified insufflation gas reduces postoperative pain and reduces postoperative recovery time.6,7 This study reaches the same conclusions by using a device that combines the benefits of both heating and hydrating the gas. Exactly how the use of heated and hydrated gas reduces pain is not clear. It is postulated that the loss of peritoneal integrity due to dessication results in release of chemical agents responsible for pain perception. Temperature-sensitive transmitters may additionally account for the loss of integrity of temperature regulation and pain sensation. Further studies are necessary to elucidate the etiology of these effects and the benefits afforded by gas pre-conditioning.
Since the advisability of a procedure or intervention is a scientific judgement, clinical effectiveness is necessary. The effectiveness of heated hydrated gas is demonstrated in this and other studies. It is also important to evaluate economic utility as a factor in making medical judgements. Analysis of outcomes from previous studies show that the use of the conditioned gas at laparoscopy favorably influences outcomes (reducing pain and rapid recovery and return to work).6,7 Use of the Insuflow® device was less expensive in this study and resulted in better outcomes than using the current standard raw gas.
Economic consequences (costs) of hypothermia have been previously reported.27 These include increased time in the recovery area, use of alternative warming methods and increased need for analgesic medicines.6 The financial penalty for not using pre-conditioned gas includes time, equipment, use of modifying techniques that only partially treat the effect and not the cause and slower return to full function. The economic savings per procedure using the Insuflow® device on every laparoscopy far exceeds the costs of the current methods it replaces, making it clearly a better strategy. There is decreased need to de-fog the laparoscope lens because the intra-abdominal dew point is not reached, eliminating the need for de-fogging agents. Time, operating room costs and frustration are saved with not having to de-fog. Hypothermia is reduced or eliminated, making surface warming methods less necessary. Recovery room time is decreased as a result of the patients' more euthermic postoperative state. The need for postoperative analgesic medicines is reduced because of significantly reduced pain perception. Return to full function activities is improved by over 50%.
In summary, laparoscopic procedures that induce hypothermia are favorably influenced by heated hydrated gas and also reduce pain and shorten recovery room stay significantly. Laparoscopic procedures that minimally affect hypothermia reduce postoperative pain and shorten recovery room stay when heated hydrated gas is used. The Insuflow® device pre-conditions laparoscopic gas by heating and hydration, significantly reduces with regard to incidence, severity and extent of postoperative pain, shortens recovery room length of stay and reduces hypothermia by maintaining a more normal physiologic intra-abdominal state. The safety profile of heated hydrated gas exceeds that of current standard raw gas. The use of heated hydrated gas during laparoscopy is a significant breakthrough that is beneficial to the patient's outcome.
Footnotes
The Insuflow® Study Group includes the following investigators: Harry Reich, MD, Columbia-Presbyterian-Sloan Hospital, Columbia University, New York; C.Y. Lui, MD, Chattanooga Women's Center, Chattanooga, Tennessee; Andrew Toledo, MD, Reproductive Biology Associates, Atlanta, Georgia; Kalyani Kumar, MD, Capital Hospital, Richmond, Virginia; and Radha Syed, MD, Staten Island University Hospital, Staten Island, New York.
Georgia BioMedical, Inc. supplied the Insuflow® devices at no cost to institutions or patients. Insuflow® and CO2 Guard® are marketed by Georgia BioMedical, Inc.
References:
- 1. Ott DE. Contamination via gynecologic endoscopy insufflation. J Gyn Surg. 1989;5:205–208 [Google Scholar]
- 2. Bessell JR, Karatassas A, Patterson JR, Jamieson GG, Maddern GJ. Hypothermia induced by laparoscopic insufflation. Surg Endosc. 1995;9:791–796 [DOI] [PubMed] [Google Scholar]
- 3. Bessell JR, Maddern GJ. Influence of gas temperature during laparoscopic procedures. In Rosenthal RJ, Friedman RL, Phillips EH. eds. The Pathophysiology of Pneumo-peritoneum. Berlin: Springer-Verlag; 1998 [Google Scholar]
- 4. Ott DE. Correction of laparoscopic insufflation hypothermia. J Laparoendosc Surg. 1991;1:183–186 [DOI] [PubMed] [Google Scholar]
- 5. Ott DE. Laparoscopic hypothermia. J Laparoendosc Surg. 1991;1:127–131 [DOI] [PubMed] [Google Scholar]
- 6. Korell M, Schmaus F, Strowitzki T, Schneeweiss SG, Hepp H. Pain intensity following laparoscopy. Surg Laparosc Endosc. 1996;6:375–379 [PubMed] [Google Scholar]
- 7. Mouton WG, Bessell JR, Millard SH, Maddern GL. A randomized controlled trial assessing the benefit of humidified insufflation gas during laparoscopic surgery. Rome Abstract World Congress Endoscopy to be published in Endoscopic Surgery. [DOI] [PubMed] [Google Scholar]
- 8. Semm K, Arp WD, Trappe M, Kube D. Pain reduction after pelvi-laparoscopic interventions by insufflation of CO2 gas at body temperature. Gerburts Frauen. 1994;54:300–304 [DOI] [PubMed] [Google Scholar]
- 9. Beery H, Husskinson EC. Measurement of pain. J Clin Trials. 1972;9:13–18 [Google Scholar]
- 10. United States Pharmacopeia and National Formulary and Supplements, XXI-NF, 1984 [Google Scholar]
- 11. Diamond MP. Adhesion prevention. In Gershenson DM, DeCherney AH, Curry SL. eds. Operative Gynecology. Philadelphia: WB Saunders Company; 1993:147–158 [Google Scholar]
- 12. DiZerga GS. The peritoneum and its response to surgical injury. In DiZerga GS, Malinak LR, Diamond MP, Linsky CB. eds. Treatment of Post Surgical Adhesions. New York: Wiley-Liss; 1990 [Google Scholar]
- 13. Ott DE. Microbial colonization of laparoscopic gas delivery systems: a quantitative analysis. JSLS. 1997;1:325–329 [PMC free article] [PubMed] [Google Scholar]
- 14. Couper GW, Ewen SW, Krukowski ZH. Risk of contamination from laparoscopic carbon dioxide insufflators. J R Coll Surg Edinb. 1997;42:231–232 [PubMed] [Google Scholar]
- 15. Ryan GB, Groberty J, Majno G. Mesothelial injury and repair. Am J Path. 1973;71:93–112 [PMC free article] [PubMed] [Google Scholar]
- 16. Gray RI, Henderson AC, Ott DE. Severe local hypothermia from laparoscopic gas, evaporative jet cooling. Ann Biomed Engineering. 1997;25:Suppl 1, Abstract 28 [PMC free article] [PubMed] [Google Scholar]
- 17. Morris RH, Kumar A. The effect of warming blankets on maintenance of body temperature of the anesthetized, paralyzed adult patient. Anesthesiology. 1970;36:408–411 [DOI] [PubMed] [Google Scholar]
- 18. Carli F, Emery PW, Freemantle CAJ. Effect of perioperative normothermia on postoperative protein metabolism. Br J Anesth. 1989;63:276–282 [DOI] [PubMed] [Google Scholar]
- 19. Michelson AD, MacGregor H, Barnard MR, Kestin AS, Roher MJ, Valeri CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Throm Haemost. 1994;71:633–640 [PubMed] [Google Scholar]
- 20. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. NEJM. 1996;334:1209–1215 [DOI] [PubMed] [Google Scholar]
- 21. Lenhardt R, Kurz A, Sessler DI, Marker E, Narzt E, Lackner F. Intra-operative hypothermia prolongs duration of postoperative recovery. Anesthesiology. 1995;83:Suppl:A1114 abstract [DOI] [PubMed] [Google Scholar]
- 22. Lenhardt R, Marker E, Goll V, Tschernich H, Sessler DI, Narzt E, Lackner F. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997;87:1318–1323 [DOI] [PubMed] [Google Scholar]
- 23. Frank SM, Higgins MS, Breslow MJ, et al. The catecholamine, cortisol and hemodynamic responses to mild perioperative hypothermia: a randomized clinical trial. Anesthesiology. 1995;82:83–93 [DOI] [PubMed] [Google Scholar]
- 24. Kurz A, Sessler DI, Nartz E, et al. Postoperative hemodynamic and thermoregulatory consequences of intra-operative core hypothermia. J Clin Anesth. 1995;73:59–7366 [DOI] [PubMed] [Google Scholar]
- 25. Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;227:1127–1134 [PubMed] [Google Scholar]
- 26. Sessler DI. Perianesthetic thermoregulation and heat balance in humans. FASEB J. 1993;7:638–644 [DOI] [PubMed] [Google Scholar]
- 27. Conahan TJ. Heating reduces recovery time (cost) in out-patients. Anesthesiology. 1982;67:128–130 [DOI] [PubMed] [Google Scholar]