Abstract
Background and Objectives:
During laparoscopy, the increase of the carbon dioxide tension may increase the synthesis of hydrochloric acid in the parietal cells of the stomach; the source of the secreted hydrogen ions is carbonic acid derived from the hydration of carbon dioxide. The present report tests this hypothesis by correlating the changes of end-tidal PCO2 (ETCO2) with the pH of the gastric juice in patients undergoing laparoscopic cholecystectomy.
Methods:
40 adult patients were investigated: 20 controls, and 20 patients receiving 100 mg nizatidine intravenously, prior to surgery. In both groups, the ETCO2 was measured by capnography and the pH of the gastric juice was monitored before carbon dioxide insufflation and at the end of laparoscopy prior to carbon dioxide deflation.
Results:
In the control group, the ETCO2 increased following carbon dioxide insufflation from a mean basal value of 30.2 (standard deviation [SD] 4.6) mm Hg to 41.1 (SD 9.5) mm Hg, while the mean pH of the gastric juice decreased significantly from 1.9 (SD 0.4) to 1.27 (SD 0.43). There was a significant negative correlation between the ETCO2 and pH of the gastric juice (r=-0.4). In the Nizatidine group, the ETCO2 also increased following carbon dioxide insufflation from a mean basal value of 30.9 (SD 3.0) mm Hg to 39.4 (SD 5.3) mm Hg. However, in contrast with the control group, the mean pH of the gastric juice did not decrease, but paradoxically increased from 1.68 (SD 0.36) to 3.6 (SD 1.02).
Conclusions:
During laparoscopy, the pH of the gastric juice is significantly decreased. This decrease is inversely related to the increase of ETCO2. The preoperative administration of the selective H2-blocker nizatidine can prevent the increase in gastric acidity and can result in a paradoxical increase of pH of the gastric juice.
Keywords: Laparoscopy, Cholecystectomy, Carbon dioxide, Capnography, H2-blocker, Nizatidine, Gastric juice, HC1
INTRODUCTION
During laparoscopic surgery, pneumoperitoneum is induced by insufflation of carbon dioxide. Keeping minute ventilation volume constant can therefore result in an increase of the arterial PCO2, with a corresponding increase of the end-tidal PCO2 (ETCO2).1–3
Carbon dioxide is involved in the synthesis of hydrochloric acid in the parietal cells of the stomach; the course of the secreted hydrogen ion is carbonic acid derived from the hydration of carbon dioxide.4,5 Thus, we postulate that the increase of carbon dioxide tension during laparoscopy may increase the acidity of the gastric juice. The present report tested this hypothesis in patients undergoing laparoscopy by correlating the changes of ETCO2 following carbon dioxide insufflation with the changes of the pH of the gastric juice. The report also investigated the possible prophylactic effect of nizatidine (Axid®), a new selective H2 receptor antagonist,6–8 on the changes of the pH of the gastric juice.
MATERIALS AND METHODS
The study was approved by the Institution Ethics Committee, and consent was obtained from all patients. We prospectively studied 40 adult patients, ASA I-II, undergoing elective laparoscopic cholecystectomy. Patients with known sensitivity to the H2-receptor antagonist and those receiving antacids or drugs known to affect pH of the gastric juice were not included in this study. Also, we excluded patients with previous ulcer symptoms, gastroesophageal reflux, or hiatus hernia.
All patients were fasted overnight and premedicated with intramuscular atropine 0.6 mg, meperidine 1-2 mg/kg and promethazine 25 mg one hour prior to induction of anaesthesia. Patients were allocated randomly to one of two groups, each consisting of 20 patients. Group I (the control group) received no H2-receptor antagonist prior to induction, while group II received nizatidine (Axid®) 100 mg intravenously, 20 minutes prior to induction of anaesthesia.
In both groups, anaesthesia was induced with thiopen-tone 4-6 mg/kg, fentanyl 3 (g/kg, and vecuronium 0.1 mg/kg. The trachea was intubated, and anaesthesia was maintained with 66% N2O in O2 supplemented with isoflurane and incremental doses of vecuronium. Routine monitoring consisted of electrocardiogram, non-invasive blood pressure measurement, as well as pulse oxymetry and capnography (Hewlett Packard). Immediately after induction of anaesthesia and tracheal intubation, a 16-F gauge nasogastric tube was inserted into the stomach and its correct position was checked by auscultation of injected air. Ventilation using a carbon dioxide absorption circuit was controlled throughout surgery at a rate of 10-12 breathmin-1, and the exhaled tidal volumes ranged between 10 and 12 ml-kg-1, as checked by Ohmeda 5400 volume monitor. The minute volume and rate of ventilation used in each patient were maintained constant throughout the laparoscopic procedure. The ETCO2 was continuously monitored by capnography with the sampling sensor located between the tracheal tube connector and the anaesthesia circuit. After 20 minutes of steady ventilation, a baseline value of the ETCO2 and the pH of the gastric juice was obtained prior to insufflation of carbon dioxide. A Veress needle was then introduced into the abdominal cavity via a 1 cm supraumbilical incision, and carbon dioxide was insufflated into the peritoneal cavity to maintain an intra-abdominal pressure ranging from 10 to 15 mm Hg. At the end of laparoscopy and prior to carbon dioxide deflation, the ETCO2 and pH of the gastric juice were recorded.
All data are presented as mean and standard deviation (SD). Comparison between the two groups of patients for all variables including demographic data, ETCO2 and pH of the gastric juice measurement were made with a two-sample t-test. Within each group, comparisons were per-formed between the preinflation measurements and the predeflation measurements using the paired t-test. Also, the individual end-tidal PCO2 values were correlated with the pH of the gastric juice. A value of P<0.05 was considered statistically significant.
RESULTS
The two groups of patients were comparable in age, weight, sex, fasting time and duration of surgery (Table 1). The end-tidal PCO2 values and the pH of the gastric juice before and after carbon dioxide insufflation in both the control group and the nizatidine group are shown in Table 2.
Table 1.
Demographic data.
| N | Age (years) | Body weight (kg) | Duration (minutes) | Fasting time (hrs) | Male/female | |
|---|---|---|---|---|---|---|
| control group | 20 | 49 | 82.7 | 81.25 | 10.6 | 11/9 |
| nizatidine group | 20 | 46.9 | 81.8 | 72.5 | 10.07 | 12/8 |
Table 2.
The end tidal PCO2 (ETCO2) and the pH of the gastric juice in both the control and the nizatidine groups, before carbon dioxide insufflation (preinflation) and following carbon dioxide insufflation (predeflation).
| CONTROL GROUP | NIZATIDINE GROUP | |||||||
|---|---|---|---|---|---|---|---|---|
| Preinflation | Predeflation | Preinflation | Predeflation | |||||
| patient | pH | ETCO2 (mm Hg) | pH | ETCO2 (mm Hg) | PH | ETCO2 (mm Hg) | pH | ETCO2 (mm Hg) |
| 1 | 1.71 | 24 | 1.02 | 29 | 1.8 | 31 | 3.9 | 39 |
| 2 | 1.85 | 26 | 1.4 | 32 | 1.79 | 28 | 2.46 | 32 |
| 3 | 2.59 | 31 | 1.29 | 34 | 1.49 | 33 | 1.98 | 40 |
| 4 | 1.3 | 26 | 0.92 | 42 | 1.6 | 31 | 4.42 | 35 |
| 5 | 1.84 | 27 | 1.5 | 34 | 2.04 | 35 | 3.66 | 38 |
| 6 | 2.76 | 26 | 2.58 | 31 | 2.22 | 32 | 6.8 | 39 |
| 7 | 1.82 | 25 | 0.92 | 35 | 1.69 | 25 | 3.6 | 36 |
| 8 | 1.8 | 33 | 0.96 | 39 | 1.84 | 27 | 3.13 | 34 |
| 9 | 2.12 | 26 | 2.05 | 29 | 1.38 | 29 | 4.77 | 37 |
| 10 | 1.51 | 34 | 1.21 | 61 | 1.46 | 27 | 3.57 | 33 |
| 11 | 2.5 | 35 | 1.17 | 55 | 1.7 | 30 | 2.8 | 33 |
| 12 | 1.91 | 34 | 0.6 | 44 | 1.02 | 29 | 4.2 | 43 |
| 13 | 1.08 | 26 | 1.01 | 41 | 1.2 | 35 | 3.2 | 52 |
| 14 | 1.59 | 25 | 1.5 | 34 | 2.49 | 34 | 4.12 | 43 |
| 15 | 1.57 | 32 | 1.08 | 40 | 1.66 | 35 | 2.98 | 47 |
| 16 | 2.7 | 29 | 1.36 | 42 | 2.15 | 32 | 3.26 | 42 |
| 17 | 1.9 | 36 | 1.7 | 52 | 2.01 | 34 | 4.72 | 45 |
| 18 | 2.11 | 36 | 0.96 | 45 | 1.22 | 29 | 3.3 | 41 |
| 19 | 1.86 | 32 | 0.91 | 42 | 1.5 | 35 | 2.5 | 46 |
| 20 | 2.27 | 40 | 1.38 | 61 | 1.43 | 28 | 3.87 | 33 |
| Mean | 1.9395 | 30.15 | 1.276 | 41.1 | 1.6845 | 30.95 | 3.662 | 39.4 |
| SD | 0.439528 | 4.596466 | 0.438605 | 9.491575 | 0.361462 | 3.057368 | 1.024732 | 5.351635 |
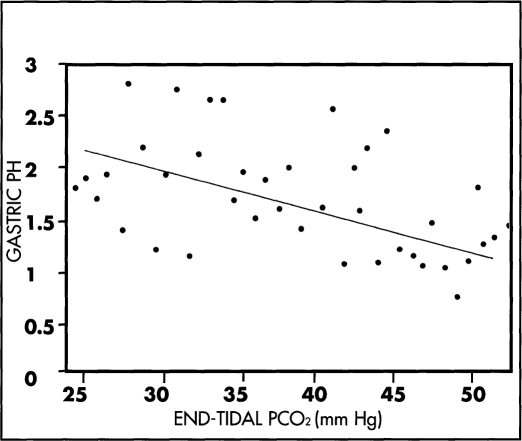
In the control group, the mean ETCO2 prior to carbon dioxide insufflation was 30.2 (SD 4.6) mm Hg and was increased to 41.1 (SD 9.5) mm Hg following insufflation (P<0.001), while the mean pH of the gastric juice was decreased from 1.93 (SD 0.43) to 1.27 (SD 0.43) (P<0.001). Correlation of the individual end-tidal PCO2 values with the pH of the gastric juice shows a significant negative correlation (Figure 1).
Figure 1.
Correlating between the ETCO2 and the pH of gastric juice in the control 20 patients. There is a significant negative correlation (r = -0.4, P< 0.01).
In the nizatidine group, the mean ETCO2 prior to carbon dioxide insufflation was 30.95 (SD 3.05) mm Hg and was increased up to 39.4 (SD 5.35) mm Hg following insufflation (P<0.001), while the mean pH of the gastric juice was significantly increased from 1.68 (SD 0.36) to 3.66 (SD 1.02) (PO.001).
Comparing group I to group II, the mean ETCO2 prior to carbon dioxide insufflation was 30.15 (SD 4.59) mm Hg in the control group and 30.95 (SD 3.05) mm Hg in the nizatidine group (P>0.05), while the mean pH of the gastric juice was respectively 1.93 (SD 0.43) and 1.68 (SD 0.36) (P>0.05). Following carbon dioxide insufflation, the mean ETCO2 was 41.1 (SD 9.49) mm Hg in the control group and 39.4 (SD 5.35) in the nizatidine group (P>0.05), while the mean pH of the gastric juice was respectively 1.27 (SD 0.43) and 3.66 (SD 1.02) (PO.001).
DISCUSSION
During laparoscopic surgery, insufflation of carbon dioxide into the peritoneal cavity creates a high carbon dioxide tension gradient between the pneumoperitoneum and the blood perfusing the peritoneum. The carbon dioxide can be readily absorbed into the blood stream. The arterial PCO2 is determined by the ratio of carbon dioxide production/alveolar ventilation. Following carbon dioxide insufflation during laparoscopy, the arterial PCO2 is determined by the ratio of alveolar ventilation to a combination of the endogenous carbon dioxide production, as well as carbon dioxide absorbed from the peritoneal cavity.9 Keeping minute ventilation constant can therefore result in an increase of the arterial PCO2 with a corresponding increase of the ETCO2.1,2
Our study shows that in patients undergoing laparoscopic cholecystectomy, carbon dioxide insufflation in the control group is followed by a significant increase of ETCO2, associated with a significant decrease of the pH of the gastric juice. Correlation of the individual pH of the gastric juice with the ETCO2 shows that the pH of the gastric juice change is inversely related to ETCO2. In view of the significant negative correlation between the ETCO2 and the pH of the gastric juice, it is suggested that the increased carbon dioxide tension during laparoscopy may be one of the factors contributing to the increased gastric acidity. Thus, it is recommended that moderate hyper-ventilation must be achieved during laparoscopy to avoid the increase of arterial carbon dioxide tension,1,2 with the consequent decrease of the pH of gastric juice.
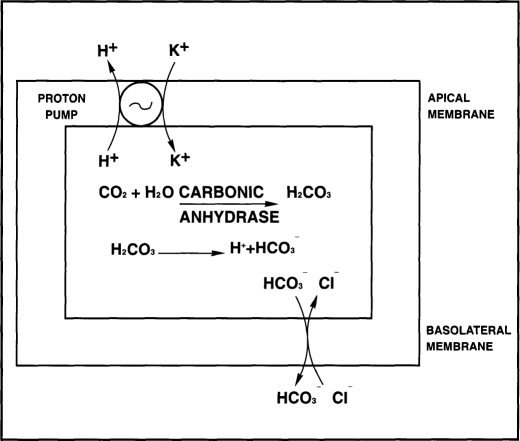
In the stomach, similar to the kidney, the source of the secreted hydrogen ions is carbonic acid derived from the hydration of carbon dioxide. In the parietal cells of the stomach, H2O generated intracellularly combines with CO2 to form H2CO3. This reaction can occur under baseline conditions, but it can be accelerated 5000 times by carbonic anhydrase, an enzyme present in abundance in the parietal cells. H2CO3 then dissociates to H+ and HCO3-. The proton pump extrudes H+ in exchange for K+, while HCO3- is secreted at the basolateral side in exchange for Cl- which is required for HCl formation4,5 (Figure 2). During laparoscopy, increased concentration of carbon dioxide can rapidly diffuse into the gastric mucosa. It may be reasonable to postulate that this excess of CO2 is utilized by the parietal cell, which converts it to HCl through the pivotal step mediated by carbonic anhydrase. The decrease of pH of the gastric juice in our patients is inversely related to the increase of ETCO2.
Figure 2.
Diagram illustrating the steps of hydrochloric acid secretion by the parietal cells of the stomach. Carbon dioxide is hydrated to carbonic acid, which dissociates into H+ + HCO3-. The proton pump actively extrudes H+ in exchange for K+, while the HCO3- is secreted in exchange for HC1-.
In contrast with the control group, carbon dioxide insufflation in the nizatidine group was not followed by a decrease of the pH of the gastric juice, but by a significant increase of pH. The results suggest that the increased acidity of the gastric juice during laparoscopy can be blocked by the preoperative intravenous administration of a single dose of nizatidine (Axid®), a selective H2-antagonist.6,7
Histamine is synthesized by the enterochromaffine-like (ECL) cells and stored in acidic granules. In response to stimulation by gastrin or acetylcholine, the ECL cells degranulate and release histamine which binds to its H2-receptors on the basolateral side of the parietal cell. In response to histamine binding, the receptor undergoes a conversion of ATP to cyclic adenosine monophosphate (cAMP), resulting in activation of the proton pump.5,10 H2-blockers are competitive antagonists of the parietal H2-receptors; they interfere with the production of cAMP secondary to H2-receptor stimulation, and thus prevent activation of the proton pump with a subsequent decrease of hydrogen ion secretion. H2-receptor antagonists are capable of reducing basal secretion of acid, as well as food, neural and hormonal-stimulated acid secretion. Nizatidine is a highly selective H2 receptor blocking drug which is characterized by high bioavailability, and short onset to peak effect.6–8 The present report shows that nizatidine in patients undergoing laparoscopic surgery can prevent any decrease of the pH of the gastric juice following carbon dioxide insufflation, and can even produce an increase of the pH of the gastric juice.
In conclusion, during laparoscopic surgery, carbon dioxide insufflation can result in a significant increase of the ETCO2 associated with a significant decrease of the pH of the gastric juice. The decrease in pH of the gastric juice is inversely related to the increase of ETCO2. Thus, moderate hyperventilation is recommended during laparoscopic surgery. Also, a selective H2-blocker may be administered prophylactically to prevent this decrease in the pH of gastric juice.
References:
- 1. Baraka A, Jabbour S, Hammoud R, et al. Can pulse oximetry and dioxide elimination during laparoscopic cholecystectomy reflect arterial oxygenation and carbon dioxide elimination during laparoscopic cholecystectomy? Surg Laparosc Endosc. 1994;4(4):353–356 [PubMed] [Google Scholar]
- 2. Baraka A, Jabbour S, Hammoud R, et al. End-tidal carbon dioxide tension during laparoscopic cholecystectomy. Anaesth. 1994;49:304–306 [DOI] [PubMed] [Google Scholar]
- 3. Wahba RWM, Mamazza J. Ventilatory requirements during laparoscopic cholecystectomy. Can J of Anaesth. 1993;40:206–210 [DOI] [PubMed] [Google Scholar]
- 4. Forte JG, Machen TE, Obrink KJ. Mechanisms of gastric H+ and Cl-transport. Ann Rev Physiol. 1980;42:111. [DOI] [PubMed] [Google Scholar]
- 5. Goldschmiedt M, Feldman M. Gastric secretion in health and disease. In Sleisenger MH, Fordtran JS. (eds). Gastrointestinal Disease: Pathophysiology, Diagnosis and Management. Fifth Edition. W.B. Saunders Company: Philadelphia; Vol 1, 1993:524–544 [Google Scholar]
- 6. Mikawa K, Nishina K, Maekawa N, Takao Y, Obara H. Effects of oral nizatidine on preoperative gastric fluid pH and volume in children. Br J Anaesth. 1994;73:600–604 [DOI] [PubMed] [Google Scholar]
- 7. Gallaghan JT, Bergstrom RF, Rubin A, et al. A pharmacokinetic profile of nizatidine in man. Scand J Gastroenterol. 1987;22:(Supp 136):9–17 [DOI] [PubMed] [Google Scholar]
- 8. Popat MT, Dyar OJ, Blogg CE. Comparison of the effects of oral nizatidine and ranitidine on gastric value and pH in patients undergoing gyneacological laparoscopy. Anaesth. 1991;46(10):816–819 [DOI] [PubMed] [Google Scholar]
- 9. Tan RL, Lee TL, Tweed WA. Carbon dioxide absorption and gas exchange during pelvic laparoscopy. Can J Anaesth. 1992;39:677–681 [DOI] [PubMed] [Google Scholar]
- 10. Gantz I, Del Valle J, Wang TS, et al. Molecular basis for the interaction of histamine with the histamine H2 receptor. J Biol Chem. 1992;267(29):20840–20843 [PubMed] [Google Scholar]