Abstract
Ca2+ sensitization of smooth muscle contraction involves inhibition of myosin light chain phosphatase (SMPP-1M) and enhanced myosin light chain phosphorylation. Inhibition of SMPP-1M is modulated through phosphorylation of the myosin targeting subunit (MYPT1) by either Rho-associated kinase (ROK) or an unknown SMPP-1M-associated kinase. Activated ROK is predominantly membrane-associated and its putative substrate, SMPP-1M, is mainly myofibrillar-associated. This raises a conundrum about the mechanism of interaction between these enzymes. We present ZIP-like kinase, identified by “mixed-peptide” Edman sequencing after affinity purification, as the previously unidentified SMPP-1M-associated kinase. ZIP-like kinase was shown to associate with MYPT1 and phosphorylate the inhibitory site in intact smooth muscle. Phosphorylation of ZIP-like kinase was associated with an increase in kinase activity during carbachol stimulation, suggesting that the enzyme may be a terminal member of a Ca2+ sensitizing kinase cascade.
The major mechanism (1–3) linking transients in [Ca2+]i to force in smooth muscle is by phosphorylation of the 20-kDa myosin light chain (MLC20). The level of phosphorylated myosin is controlled by two enzymes: a Ca2+-calmodulin-dependent myosin light chain kinase and a myosin light chain phosphatase (SMPP-1M) (4, 5). However, at fixed Ca2+ levels contraction can also be induced by agonist stimulation or by activation of G proteins with GTPγS or AlF4 (4). This leads to so-called Ca2+ sensitization (4–7) and was shown to reflect an inhibition of SMPP-1M activity (4, 5, 8, 9).
Protein phosphatase 1 (PP-1) is one of the major Ser/Thr protein phosphatases in eukaryotic cells, and different forms of PP-1 are composed of a catalytic subunit and different regulatory subunits that target the phosphatase to specific locations and particular substrates (10–12). SMPP-1M is composed of three subunits: the 37-kDa catalytic subunit of PP-1 (PP1Cδ); a 110–130-kDa regulatory myosin phosphatase targeting subunit (MYPT1); and a 20-kDa subunit of undetermined function (13–15). The myosin phosphatase activity of SMPP-1M is thought to be regulated by phosphorylation of the MYPT1 subunit. There are several phosphorylation sites on MYPT1, including an inhibitory site of phosphorylation by an endogenous kinase (16) identified as Thr695 (in the chicken MYPT1 isoform). Subsequent data indicated that there are two major sites on MYPT1 for Rho-associated protein kinase (ROK); these sites are Thr697 (numbering for rat isoform and equivalent to Thr695) and Ser854 (9, 17–19). Recently, it was shown that the site responsible for inhibition of SMPP-1M is Thr697 (18, 19). Thus, it is clear that ROK plays an important role in Ca2+-sensitization of smooth muscle. However, the finding of an additional endogenously associated MYPT1 kinase (16) and the recruitment of ROK to RhoA-GTP at the cell membrane raises both temporal and spatial concerns about access of ROK to the substrate MYPT1. To clarify this situation and to identify the endogenous or SMPP-1M associating kinase, we initiated this study.
Materials and Methods
Materials.
Affinity-purified anti-MYPT1 antibody was prepared by Quality Control Biochemicals (Hopkington, MA). Anti-ZIP kinase (ZIPK) antibody was from Calbiochem. Gamma-linked ATP Sepharose was produced in our laboratory (20). Bovine brain ROK was a gift of Michael Walsh (University of Calgary). ROK inhibitor, Y-27632, was a gift from Mitsuisa Yoshimura (Welfide Corporation). Two recombinants based on the chicken MYPT1 isoforms (M130 and M133) were prepared as described (21, 22). Thr697 substrate peptide (KKKRQSRRSTQGVTL) containing Arg690 to Lys701 of MYPT1 was synthesized by Biomolecules Midwest (St. Louis). 32P-labeled myosin and glycogen phosphorylase a were prepared as described (13).
Kinase and Phosphatase Assays.
Kinase assays included 10 μl of enzyme diluted in 25 mM Hepes, pH 7.4, 1 mM DTT, and 100 μM Thr697 peptide. Reactions were started with addition of 20 μl Mg2+ ATP [5 mM MgCl2 and 0.1 mM ATP (5,000 cpm/nmol)] and carried out at 25°C. Reactions were terminated after 20 min with the addition of 100 μl of 20 mM H3PO4. Aliquots (100 μl) of the reaction mixture were spotted onto P81 paper and washed four times with 20 mM H3PO4. The P81 paper was placed into 1.5-ml Eppendorf tubes and 32P incorporation was determined by scintillation counting. Phosphatase assays were carried out as described (13).
In-Gel Kinase Assay.
In-gel kinase assays were performed as described (23). Samples containing kinase activity were boiled (5 min) in sample buffer and separated in SDS/PAGE gels (10%) containing Thr697 peptide (0.5 mg/ml). After electrophoresis, the gels were incubated in 20% isopropanol containing 50 mM Hepes, pH 7.5, twice for 30 min, and washed in 50 mM Hepes, pH 7.5, containing 5 mM 2-mercaptoethanol. After denaturation with 6 M guanidine-HCl, 5 mM 2-mercaptoethanol and 50 mM Hepes, pH 7.5, the kinases in the gels were renatured (5°C) by incubation in successive dilutions of guanidine-HCL (3, 1.5, 0.75, and 0 M), 0.05% Tween-20, and 5 mM 2-mercaptoethanol for 45 min each. For the kinase reaction, the gels were equilibrated for 30 min in kinase buffer (50 mM Hepes, pH 7.5, 0.1 mM EGTA, 20 mM MgCl2, and 2 mM DTT) before incubation with 25 μM [γ-32P]ATP (1 μCi/μM; 1 Ci = 37 GBq). The reaction was terminated by washing the gels in 5% TCA/1% sodium pyrophosphate. The gels were dried and autoradiographed.
Purification of the SMPP-1M-Associated Kinase.
The SMPP-1M-associated kinase was isolated from cow bladders following initial steps outlined for purification of SMPP-IM from pig bladder (13). Following extraction of the myofibrillar pellet, the extract was diluted with 2 volumes of buffer C (20 mM Tris, pH 7.5, 25 mM MgCl2, and 1 mM DTT with protease inhibitors), clarified by centrifugation (100,000 g, 45 min), and applied to a 5.0 cm × 10 cm column of ethylenediamine γ-linked ATP Sepharose equilibrated in buffer C. The column was washed with buffer C, and then buffer C containing 100 μM geldanamycin to eliminate recovery of HSP90 (P. Fadden and T.A.J.H., unpublished data). Kinase activity was eluted in 5-ml fractions with 20 mM ATP in buffer C. Active fractions were pooled, dialyzed against buffer D (20 mM Tris, pH 8.0, 1 mM EDTA, 1 mM DTT) and applied to an AP-1Q anion exchange column (1.5 cm × 10 cm) equilibrated in buffer D. The column was washed with buffer D and developed with a 0–1-M salt gradient. Fractions were assayed for SMPP-1M kinase activity. Active fractions were pooled, dialyzed against buffer E (20 mM Tris, pH 7.5, 10 mM MgCl2, 1 mM DTT) and applied to a Cibicron Blue 3GA column (1.5 cm × 10 cm) equilibrated in buffer E. The column was developed with a 0–2-M NaCl gradient; fractions containing SMPP-1M kinase activity were pooled and dialyzed against buffer D. Following concentration (2 ml) the pool was applied to a SMART Mono-Q PC 1.6/5 column. Fractions (50 μl) were assayed for SMPP-1M kinase activity. The purity of SMPP-1M kinase was assessed by SDS/PAGE and silver staining.
Mixed Peptide Sequencing.
Fractions containing SMPP-1M kinase activity were separated by SDS/PAGE and electroblotted to polyvinyl membrane. The transferred proteins were stained with Amido Black and identified by mixed peptide sequences as described (24).
Preparation of Recombinant Glutathione S-Transferase (GST)-ZIPK Fusion Proteins.
The GenBank dbEST database was searched with the complete sequence of human ZIPK. image cDNA clones AI660136 (1–955 bp) and AW237698 (19–930 bp) encoding the N-terminal(1–320) portion of ZIPK were obtained from Genome Systems (St. Louis). Both clones are 99.9% homologous to the N-terminal domain of human ZIPK (34). cDNA clones were in-frame inserted into vector pGEX-4T-1 (Amersham Pharmacia) to express the GST fusion protein. Escherichia coli cells were cultured in LB broth, 50 μg/ml ampicillin, overnight at 37°C. Cells were induced with 100 μM isopropyl-β-d-thiogalactopyranoside, and GST-ZIK isolated by using glutathione-Sepharose 4B beads.
Immunoprecipitation Techniques.
For ZIP-like kinase coimmunoprecipitation experiments, tissue homogenates (1:5 wt/vol) from rabbit bladder were prepared in 25 mM Hepes, pH 7.5, 0.1 mM EGTA, 0.1 mM EDTA, 1 mM DTT, 0.5% Triton X-100, 600 mM NaCl, and protease inhibitors. Homogenates were centrifuged for 10 min (10,000 × g); the supernatant was removed, diluted 5-fold with buffer A, and precleared with protein A Sepharose beads (1 h at 5°C). Tissue extract was incubated overnight with 10 μg rabbit polyclonal anti-ZIPK, followed by harvest with protein A Sepharose. Immunoprecipitated proteins were resolved by SDS/PAGE, transferred to poly(vinylidene difluoride) (PVDF) membrane, and immunoblotted with rabbit anti-MYPT1 antibody. The membranes were developed by using enhanced chemiluminescence (Amersham Pharmacia). For MYPT1 coimmunoprecipitation experiments, tissue homogenates from rabbit bladder were prepared as detailed above. The extract was incubated overnight with 10 μg rabbit polyclonal anti-MYPT1, followed by harvest with protein A Sepharose. SDS/PAGE and ZIPK immunoblots were performed as above.
[32P]Orthophosphate Labeling of Rabbit Bladder.
Rabbit bladder was removed from rabbits anaesthetized with halothane
according to approved protocols. Two groups of intact smooth muscle
sheets (8 mm × 8 mm) were incubated in Hepes-buffered Krebs
solution in the presence of
[32P]PO (5 mCi/ml) at
25°C for 1 h. To inhibit endogenous phosphatase
activity, muscle pieces were treated first with calyculin A (10 μM),
then vehicle (control) or carbachol (50 μM) for 15 min more. The
tissues were flash frozen in liquid N2 then homogenized in lysis buffer
(20 mM Tris⋅HCl, pH 7.5, 250 mM sucrose, 5 mM EDTA, 1 mM DTT, 10
nM microcystin, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 0.1
mM PMSF) and centrifuged (20,000 × g). The pellets
were extracted with buffer B, centrifuged, and fractionated by micro
anion-exchange chromatography using a SMART FPLC (Amersham Pharmacia).
Column fractions were assayed for ZIP-like kinase activity.
(5 mCi/ml) at
25°C for 1 h. To inhibit endogenous phosphatase
activity, muscle pieces were treated first with calyculin A (10 μM),
then vehicle (control) or carbachol (50 μM) for 15 min more. The
tissues were flash frozen in liquid N2 then homogenized in lysis buffer
(20 mM Tris⋅HCl, pH 7.5, 250 mM sucrose, 5 mM EDTA, 1 mM DTT, 10
nM microcystin, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 0.1
mM PMSF) and centrifuged (20,000 × g). The pellets
were extracted with buffer B, centrifuged, and fractionated by micro
anion-exchange chromatography using a SMART FPLC (Amersham Pharmacia).
Column fractions were assayed for ZIP-like kinase activity.
Results
Identification of MYPT1 Phosphorylation Sites in Response to Ca2+ Sensitization.
We identified through 32P-labeling of intact smooth muscle four phosphopeptides on MYPT1 whose phosphorylation state was increased in response to Ca2+-sensitizing agents such as carbachol (Fig. 1 A and B). Phosphopeptide mapping and peptide sequencing identified the major carbachol sensitive site as Thr697 on MYPT1 (Fig. 1 B and C). Furthermore, we confirm the presence of an endogenous MYPT1 kinase that copurifies and phosphorylates Thr697, inactivating SMPP-1M in vitro (Fig. 2B).
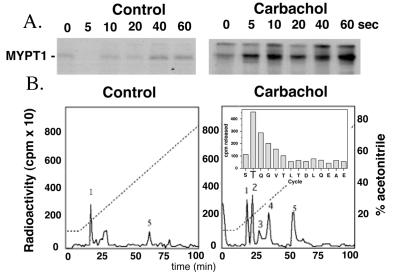
Figure 1.
Determination of the sites of phosphorylation of MYPT1 in vivo. (A) [32P]orthophosphate-labeled rabbit bladder was stimulated with 10 μM carbachol for the indicated times. MYPT1 was immunoprecipitated from tissue homogenates then resolved by SDS/PAGE. Increased MYPT1 phosphorylation was determined by autoradiography. (B) MYPT1 immunoprecipitated from control and carbachol stimulated rabbit bladder was digested overnight with trypsin; the 32P-labeled peptides obtained were separated on a C18 reverse-phase column and identified by scintillation counting. (Inset) One of the phosphorylated MYPT1 peptides (no. 2) was sequenced and its phosphorylation site identified as described (32).
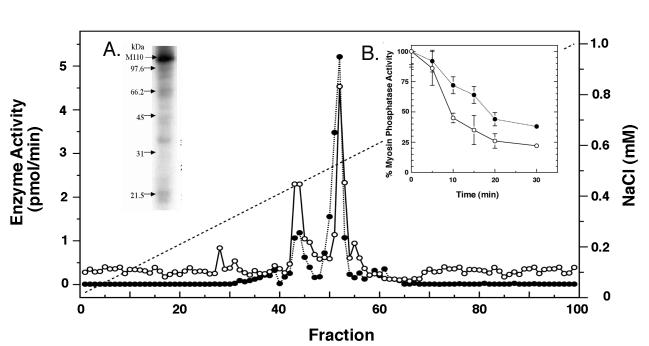
Figure 2.
Endogenous kinase copurifies with SMPP-1M. (Inset in A) Autoradiography of purified SMPP-1M shows a phosphorylated band at 110 kDa, correlating with MYPT1. SMPP-1M was affinity purified as described (13) and the purified enzyme incubated with 100 μM [γ-32P]ATP and 2 mM MgCl2. The reaction was terminated with sample buffer and MYPT1 resolved on SDS/PAGE gels. (B) Purified M110 kinases accelerates the rate of SMPP-1M inactivation in vitro. Purified SMPP-1M was incubated for the indicated times with Mg/ATP (2 mM/100 μM) in the presence (○) or absence (●) of affinity purified M110 kinase. Note: Inactivation of SMPP-1M in the absence of exogenously added M110 kinase was due to the presence of endogenous copurifying kinase activity. (A) The myofibrillar extract from rabbit bladder was resolved on an AP-1Q (0.5 cm × 7 cm) anion exchange column; the column was developed with a 0–1-M NaCl gradient. SMPP-1M (●) was assayed against 32P-labeled myosin and SMPP-1M kinase activity (λ) was assayed against the Thr697 substrate peptide (KKKRQSRRSTQGVTL).
Purification and Identification of the Endogenous MYPT1 Kinase.
To identify the endogenous kinase that is copurified with MYPT1 (Fig. 2), a substrate peptide with sequence corresponding to the Thr697 phosphorylation site of MYPT1 was synthesized. Kinase activity was isolated from the myofibrillar pellet of cow bladder and purified to near homogeneity by using a γ-phosphate-linked ATP-Sepharose affinity column. A single band of kinase activity toward the Thr697 peptide was identified by an in-gel kinase (1D and 2D SDS/PAGE) assay (23) at 32 kDa (Fig. 3). An identical band of kinase activity was obtained by using an in-gel kinase assay and the C-terminal fragment of MYPT1 as the substrate (data not shown). The SMPP-1M kinase at 32 kDa in the gels was identified by mixed peptide sequencing and was most similar to HeLa zipper interacting protein kinase ZIPK (Fig. 4). Furthermore, in-gel kinase analysis by 2D SDS/PAGE and mixed peptide sequencing confirmed that the 32-kDa band contained a single protein and not any other protein kinase (data not shown). A previous report (25) on full-length mammalian ZIPK indicated masses of 51.4 kDa and 52.5 kDa for the mouse and human isoforms, respectively, as compared with a mass of 32 kDa for the SMPP-1M-associated kinase identified herein. Whether the latter is a proteolyzed fragment of full-length ZIPK or is a smaller smooth muscle-specific isozyme remains to be determined. Preliminary Western blotting experiments with ZIPK antibody indicate the presence of two bands of both 58 kDa and 34 kDa (approximations) in most rat smooth muscles tested. Based on these studies the SMPP-1M-associated kinase identified herein is referred to as ZIP-like kinase.
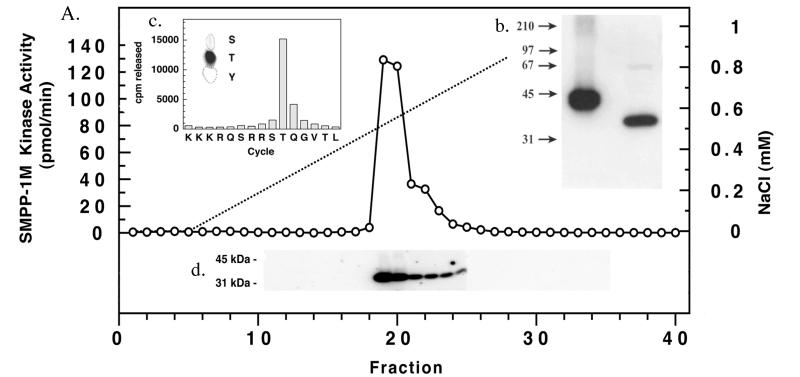
Figure 3.
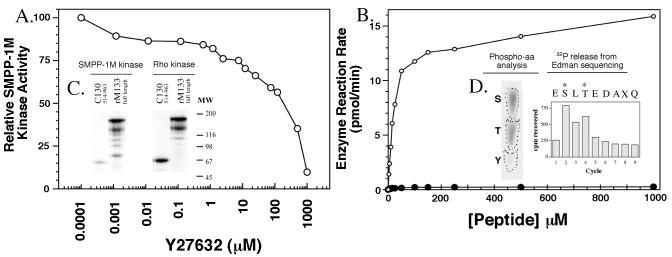
Purification of SMPP-1M-associated kinase. (A) SMPP-1M kinase was eluted from a SMART MiniQ (1.6/5) anion exchange column with a 0–1-M NaCl gradient and identified by using both in vitro and in-gel kinase assays. (Inset b) The autoradiogram of the in-gel assay localized kinase activity to a discrete protein band at 32 kDa. (Inset c) The results obtained from phosphoamino acid analysis (33) of Thr697 substrate peptide phosphorylated during the in vitro assay by purified SMPP-1M kinase. Phosphorylated Thr697 substrate peptide was sequenced and its phosphorylation site determined as described (32). (Inset d) In-gel kinase assay comparing purified PKA (1 μg) control with purified SMPP-1M kinase. A control gel run in the absence of Thr697 substrate peptide was blank.
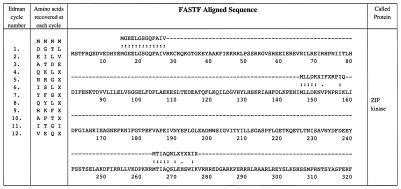
Figure 4.
Identification of SMPP-1M-associated kinase by mixed peptide sequencing. Mixed sequence is listed in order of the PTH amino acids recovered after each Edman cycle. Sequence data shown was derived from 200 fmol of protein. fastf was used to search and match the mixed sequences to the National Center for Biotechnology Information (NCBI)/Human protein database. The scoring matrix was MD20, with expectation and score values set to <1 and 5, respectively (23). The highest scoring proteins were: human ZIPK, (e) 5.1 e-14; human pDAPK3, (e) 5.1 e-14; and rat DAP-like kinase, (e) 2.1 e-7. The next highest unrelated protein score was d-glycerate dehydrogenase, (e) 0.0011.
The enzymatic properties of native (ZIP-like) and recombinant ZIPK were investigated in vitro. Recombinant 38-kDa ZIPK was expressed in E. coli and found to be constitutively active and to phosphorylate the Thr697peptide and full-length MYPT1 at Thr697 at a rate equal to that of the native purified protein. Fig. 5 shows that inhibition of native ZIP-like kinase by the ROK inhibitor Y-27632 (26) occurs at levels that are 200-fold greater than that for ROK. Recombinant ZIPK demonstrated a similar insensitivity to Y-27632. Because Y-27632 is known to inhibit ROK in vivo and brings about decreased blood pressure in hypertensive mice, the lack of sensitivity of ZIP-like kinase to the drug may suggest that the enzyme participates in a Ca2+-sensitizing signal transduction pathway downstream of ROK (26). Phosphorylation of the Thr697 peptide and full-length MYPT1 (rM133) in vitro by native ZIP-like kinase was considerably faster than by ROK (about 15-fold; Fig. 5). Interestingly, and in contrast to ROK, ZIP-like kinase more effectively phosphorylated full-length MYPT1 at Thr697 than a C-terminal fragment (residues 514–963) of the protein containing this site (Fig. 5). Recombinant ZIPK displayed identical properties (data not shown). Significantly, ZIP-like kinase or ZIPK did not phosphorylate Ser854 on full-length MYPT1. This contrasts with ROK, which has been reported to phosphorylate both Thr697 and Ser (854 in vitro) (17). This finding indicates that Thr697 phosphorylation alone is sufficient to inhibit SMPP-1M activity. To characterize recombinant ZIPK further we determined the sites of auto phosphorylation on the enzyme. Fig. 5 also shows the sequence and identifies S (110) and T (112) as phosphorylated residues in the activation loop. This finding suggests that two phosphorylation events are required to activate ZIPK. Importantly, similar analysis on ZIP-like kinase immunoprecipitated from 32P-labeled bladder showed activation correlated with increased phosphorylation (see Fig. 7).
Figure 5.
ZIP-like kinase properties toward MYPT1. (A) Effect of ROK inhibitor Y-27632 on ZIPK and ZIP-like kinase. Kinases were assayed in vitro against the Thr697 peptide. (B) Substrate concentration dependence of purified bladder ZIP kinase (○), and ROK (●). (Inset C) Autoradiograms showing phosphorylation of chicken gizzard full-length MYPT1 (18), rM133, and chicken gizzard C-terminal fragment (C130514–963; ref. 34) by purified bladder ZIPK and ROK in vitro. Data are means ± SEM of three separate experiments. (Inset D) Identification of the autophosphorylation sites on ZIPK.
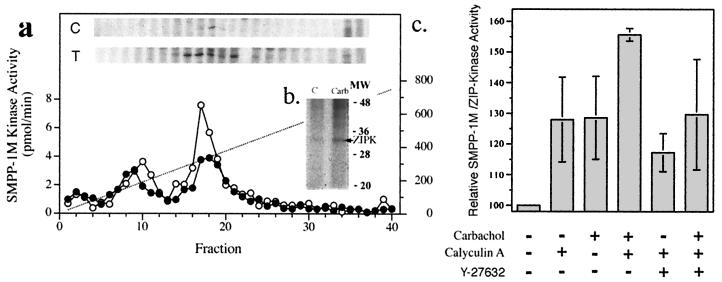
Figure 7.
Carbachol affects ZIP-like kinase phosphorylation and activity in smooth muscle. [32P]orthophosphate-labeled rabbit bladder was stimulated with 50 μM carbachol in the presence of 10 μM calyculin A. Triton-extracted tissue pellets were fractionated on a SMART MiniQ (1.6/5 cm) column. (a) Aliquots of fractions were run on SDS/PAGE gels and subjected to autoradiography (b) to visualize phosphorylation. Western immunoblots were used to identify the protein bands that corresponded with ZIPK. SMART fractions from both control (C) and carbachol-treated (T) bladder containing ZIP-like kinase were pooled, immunoprecipitated with anti-ZIP kinase antibody, and resolved on SDS/PAGE before autoradiography (b). (b) Carbachol/calyculin A treatment increase ZIP-like kinase activity. Homogenates were prepared and MYPT1 was immunoprecipitated. Immunoprecipitates were assessed in duplicate for ZIP-like kinase activity. Activity shown was derived following subtraction of nonspecific background kinase activity that was also present in the immunoprecipitate. Data represent the means ± SEM of five separate experiments. *, significantly different from the control value by the Student-Newman-Keuls test, P < 0.05; **, significantly different from the carbachol/calyculin A treatment, P < 0.05.
ZIPK and MYPT1 are Colocalized in Smooth Muscle.
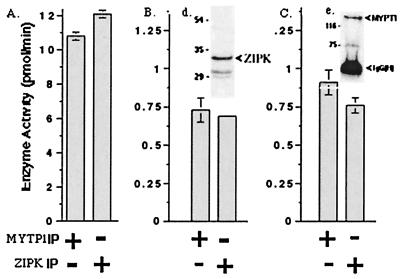
Although SMPP-1M and ZIP-like kinase copurified through three distinct chromatography steps (Figs. 2 and 3), immunoprecipitation was used to determine whether ZIP-like kinase and MYPT1 interact in smooth muscle. Immunoprecipitates of MYPT1 from rabbit bladder contained ZIPK as evidenced from immunoblotting, and similarly, when ZIP-like kinase was immunoprecipitated, MYPT1 was detected by immunoblotting (Fig. 6). ZIP-like kinase activity in MYPT1 immunoprecipitates was also measured by using the Thr697 peptide substrate by in vitro assay and by in-gel kinase assay. Kinase activity was recovered from both anti-MYPT1 and anti-ZIPK immunoprecipitates. SMPP-1M phosphatase activity in the immunoprecipitates was measured against two known SMPP-1M substrates, myosin, and glycogen phosphorylase a (13). SMPP1–1M phosphatase activity was present in the ZIP-like kinase and MYPT1 immunopellets. These experiments demonstrate that an active ZIP-like kinase is associated with fully functional SMPP-1M phosphatase in smooth muscle.
Figure 6.
Association of SMPP-1M with ZIP-like kinase. (A) MYPT1 was immunoprecipitated and ZIP-like kinase measured in the immunoprecipitate. Alternatively, ZIP-like kinase was immunoprecipitated and myosin phosphatase measured against (B) glycogen phosphorylase a or (C) myosin. (Inset d) Tissue extracts from bladder were immunoprecipitated with anti-MYPT1 antibody, resolved on SDS/PAGE, and immunoblotted for ZIPK. (Inset e) Tissue extracts from bladder were immunoprecipitated with anti-ZIPK antibody, resolved on SDS/PAGE, and immunoblotted for MYPT1.
ZIP-like Kinase Is Phosphorylated and Activated in Vivo by Carbachol.
To determine the mechanism of activation of ZIP-like kinase in vivo, the protein was immunoprecipitated from 32P-labeled rabbit bladders following treatment with the Ca2+-sensitizing drug carbachol. Treatments were carried out in the presence of calyculin A (an inhibitor of type 1A and 2A protein phosphatases) to inhibit endogenous ZIP-like kinase phosphatase activity. Fig. 7 shows that ZIP-like kinase was phosphorylated and activated in rabbit bladder smooth muscle by exposure to carbachol. In experiments carried out in the absence of calyculin A the activation of ZIP-like kinase was reduced by about 50%, indicating control of the kinase via a kinase/phosphatase couplet (Fig. 7). Phospho amino acid analysis of immunoprecipitated ZIP-like kinase from 32P-labeled bladder identified the presence of both phosphoserine and phosphothreonine (data not shown). Preliminary in vitro experiments suggest that ROK does not directly phosphorylate ZIP-like kinase indicating that additional components (such as a ZIP-like kinase kinase) may be required. Consistent with this hypothesis, treatment of carbachol- and calyculin A-treated bladder with Y-27632 (10 μM) caused a significant inhibition of ZIP-like kinase activity (Fig. 7).
Discussion
It has been shown (16) that the holoenzyme of myosin phosphatase copurified with an endogenous kinase that phosphorylated the MYPT1 subunit and inhibited phosphatase activity. From the data presented above this kinase was identified as a ZIP-like kinase. Its in vivo interaction with MYPT1 was demonstrated, and both the native (bovine) enzyme and a recombinant human fragment of ZIPK (residues 1–320) incorporating the kinase domain phosphorylated the inhibitory site on MYPT1 (i.e., Thr697). No other major site(s) of phosphorylation on MYPT1 was detected. Carbachol stimulation of the bovine bladder increased phosphorylation of the 32-kDa ZIP-like kinase and thus it is suggested that it is a component of the carbachol-induced signal transduction pathway. Although ZIP-like kinase was not directly phosphorylated and activated by ROK in vitro, treatment of bladder with Y-27632 inhibited activation of the enzyme by carbachol or calyculin A.
Ca2+ sensitization in smooth muscle, such as occurs with carbachol stimulation, involves inhibition of SMPP-1M and enhanced myosin phosphorylation at suboptimal Ca2+ concentrations (for a review, see ref. 4). Many reports have documented the important role played by RhoA in Ca2+ sensitization (for a review, see ref. 5) and its downstream partner ROK has also been implicated (9, 17, 18, 27). It is also established that ROK can directly phosphorylate MYPT1 at Thr697 (or its equivalent site in different MYPT1 isoforms) under in vitro conditions. Thus, it might be assumed that the major players in the cascade between RhoA and myosin phosphorylation are identified. However, there are concerns in accepting this as a final and obligatory scenario, and our data suggest the possibility of an additional link(s).
One potential problem with ROK being the final link to MYPT1 is the localization of the active enzyme. It has been demonstrated (28) that Ca2+ sensitization of smooth muscle involves translocation of RhoA to the membrane, where presumably it is a target for ROK. Thus, activation of ROK is thought to occur at the membrane locations and an obvious question is: How is the activated ROK transmitted to the contractile apparatus? This has not been resolved. One possibility is that ROK is autophosphorylated and activated independently of GTP-RhoA. This assumption is based largely on the precedent of the p21-activated serine/threonine kinase, where autophosphorylation of ROK has been observed (30–33), and with the isolated ROKα from chicken gizzard. In this case, it was found that autophosphorylation of ROK induces an active and RhoA-independent form. An attractive possible solution to the dilemma of separation of activated ROK and its substrate, MYPT1, is the presence of an additional link between the two. We propose that the ZIP-like kinase could serve this purpose. Whether this involves single or multiple links is not established. Because the ZIP-like kinase was not phosphorylated directly by Rho kinase in vitro it is tentatively assumed that more than one component is involved in signal transmission from ROK to MYPT1. In support of this hypothesis, we found that activation of ZIP-like kinase in vivo was blocked by Y-27632. In vitro, however, ZIP-like kinase was insensitive to the inhibitor. Finally, it should be noted that, although direct phosphorylation of MYPT1 by ROK has been demonstrated in vitro, it is not possible from the in vivo data to eliminate additional steps between ROK and MYPT1.
As described above, the ZIP-like kinase is closely related to ZIPK that in turn is related to the death-associated protein kinase (34), and the latter two are involved in the mediation of apoptosis (24, 34). Whether the ZIP-like kinase is involved in similar cell functions is not known and there are no data implicating MYPT1 in cell death. It is worth noting that ZIPK is located in the nucleus whereas ZIP-like kinase shown here is cytosolic and associated with MYPT1 and myosin. Before further speculation, it is important to establish whether the ZIP-like kinase is a truncated version of ZIPK, or an independent relative. If not identical, the two kinases must be closely related as evidenced by crossreactivity with the ZIPK antibody and similarities in enzyme properties. ZIPK contains an N-terminal catalytic domain of 263 residues and three leucine zipper sequences at its C terminus. Because the mass of the ZIP-like kinase is only approximately 32 kDa, it would contain only about 60% of the ZIPK structure. The catalytic domain would account for about 30 kDa and, thus, there would be little additional structure. For example, the leucine zipper motifs may not be present in the ZIP-like kinase. Recently there is considerable interest in the role of leucine zippers in interactions with MYPT1, because the cGMP-dependent protein kinase 1α was proposed to target MYPT1 via leucine zippers (35). It is clear, however, that leucine zipper interactions are not essential for phosphorylation of MYPT1 by ZIP-like kinase, because the chicken MYPT1 isoforms (used as substrates) do not contain leucine zippers (15). The molecular basis underlying the interaction of ZIP-like kinase with MYPT1 is not known.
In summary, the endogenous kinase associated with SMPP-1M has been identified as a ZIP-like kinase. This ZIP-like kinase phosphorylates Thr697 (Thr654, Thr695, and Thr696 in other MYPT1 isoforms) and inhibits phosphatase activity. It is suggested that the ZIP-like kinase could act downstream of RhoA and ROK in the Ca2+ sensitization mechanism in the smooth muscle. In this hypothesis, the ZIP-like kinase would act as a shuttle between the membrane-attached ROK and MYPT1 associated with the contractile apparatus. It is not known whether the ZIP-like kinase and possibly the phosphorylation of MYPT1 are involved in other cell functions.
Acknowledgments
We thank Applied Biosystems for supporting the laboratory. This work was supported in part by a Natural Sciences and Engineering Research Council of Canada (NSERC) postdoctoral fellowship (to J.A.M.) and National Institutes of Health Grants HL19242–24 and DK52378–04 (to T.A.J.H.), HL23615 (to D.J.H.), and PO1HL19242–24 (to A.V.S.).
Abbreviations
- ZIPK
ZIP kinase
- ROK
Rho-associated kinase
- MYPT1
myosin phosphatase targeting subunit
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hartshorne D J. In: Physiology of the Gastrointestinal Tract. Johnson L R, editor. New York: Raven; 1987. pp. 423–482. [Google Scholar]
- 2.Sellers J R, Adelstein R S. Curr Top Cell Regul. 1985;27:51–62. doi: 10.1016/b978-0-12-152827-0.50012-8. [DOI] [PubMed] [Google Scholar]
- 3.Somlyo A P, Somlyo A V. In: The Heart and Cardiovascular System. Fozzard H A, editor. New York: Raven; 1991. pp. 1–30. [Google Scholar]
- 4.Somlyo A P, Somlyo A V. Nature (London) 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 5.Hartshorne D J, Ito M, Erdodi F. J Muscle Res Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura J, van Breemen C. Adv Exp Med Biol. 1991;308:9–25. doi: 10.1007/978-1-4684-6015-5_2. [DOI] [PubMed] [Google Scholar]
- 7.Kitazawa T, Gaylinn B D, Denney G H, Somlyo A P. J Biol Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- 8.Somlyo A P, Kitazawa T, Himpens B, Matthijs G, Horiuti K, Koayashi S, Goldman Y E, Somlyo A V. Adv Protein Phosphatases. 1989;5:181–195. [Google Scholar]
- 9.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 10.Alms G R, Sanz P, Carlson M, Haystead T A J. EMBO J. 1999;18:4157–4168. doi: 10.1093/emboj/18.15.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard M J, Cohen P. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- 12.Egloff M P, Johnson D F, Moorhead G, Cohen P T, Cohen P, Barford D. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirazi A, Iizuka K, Fadden P, Mosse C, Somlyo A P, Somlyo A V, Haystead T A J. J Biol Chem. 1994;269:31598–31606. [PubMed] [Google Scholar]
- 14.Alessi D, MacDougall L K, Sola M M, Ikebe M, Cohen P. Eur J Biochem. 1992;210:1023–1035. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne D J, et al. J Biol Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- 16.Ichikawa K, Ito M, Hartshorne D J. J Biol Chem. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- 17.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne D J, Nakano T. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 19.Haystead C M, Gailly P, Somlyo AP, Somlyo A V, Haystead T A J. FEBS Lett. 1995;377:123–127. doi: 10.1016/0014-5793(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 20.Haystead C M, Gregory P, Sturgill T W, Haystead T A J. Eur J Biochem. 1993;214:459–462. doi: 10.1111/j.1432-1033.1993.tb17942.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Feng J, Tujino S, Inagaki N, Inagaki M, Tanaka J, Ichikawa K, Hartshorne D J, Tanabe K. Biochemistry. 1997;36:7607–7614. doi: 10.1021/bi9702647. [DOI] [PubMed] [Google Scholar]
- 22.Hirano K, Phan B C, Hartshorne D J. J Biol Chem. 1997;272:3683–3691. doi: 10.1074/jbc.272.6.3683. [DOI] [PubMed] [Google Scholar]
- 23.Kameshita I, Fujisawa H. Anal Biochem. 1989;183:139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 24.Damer C K, Partridge J, Pearson W R, Haystead T A J. J Biol Chem. 1998;273:24396–24405. doi: 10.1074/jbc.273.38.24396. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Matsumoto M, Takeda K, Sanjo H, Akira S. Mol Cell Biol. 1998;18:1642–1651. doi: 10.1128/mcb.18.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. Nature (London) 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 27.Sward K, Dreja K, Susnjar M, Hellstrand P, Hartshorne J J, Walsh M P. J Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong M C, Fujihora H, Somlyo A V, Somlyo A P. J Biol Chem. 1997;272:10704–10709. doi: 10.1074/jbc.272.16.10704. [DOI] [PubMed] [Google Scholar]
- 29.Manser E, Leung Y, Salihuddin H, Zhao Z-S, Lim L. Nature (London) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 30.Leung T, Manser E, Tan L, Lim L. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 31.Matsui T, Amano M, Yamamoto T, Kazuyasu C, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Twamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, et al. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 33.Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne D J, et al. J Biol Chem. 1999;24:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 34.Inbal B, Shani G, Cohen O, Kissil J L, Kimchi A. Mol Cell Biol. 2000;20:1044–1054. doi: 10.1128/mcb.20.3.1044-1054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surks, H. K., Mochizuki, N., Kasai, Y., Georgescu, S. P., Tang, K. M., Ito, M., Lincoln, T. M. & Mendelsohn, M. E. (1999) 286, 1583–1587. [DOI] [PubMed]