Abstract
Background:
Recent advances in laparoscopic and thoracoscopic surgery have made it possible to perform esophagectomy using minimally invasive techniques. The aim of this report was to present our preliminary experience with minimally invasive esophagectomy.
Methods:
We reviewed our experience on eight patients who underwent minimally invasive esophagectomy using either laparoscopic and/or thoracoscopic techniques from June 1996 to May 1997. Indications for esophagectomy included stage I carcinoma (5), palliative resection (1), Barrett's with high grade dysplasia (1) and end stage achalasia (1).
Results:
The average age was 68 years (54-82). The surgical approach to esophagectomy included laparoscopic transhiatal esophagectomy with cervical anastomosis (n=4), thoracoscopic and laparoscopic esophagectomy with cervical anastomosis (n=1), and laparoscopic mobilization with right mini-thoracotomy and intra-thoracic anastomosis (n=3). Conversion to mini-laparotomy was required in two patients (25%) to complete esophageal dissection and facilitate gastric pull-up. The mean operative time was 460 minutes. The mean intensive care stay was 1.9 days (range of 0-7 days) with a mean hospital stay of 13-8 days. Minor complications included atrial fibrillation (n=1), pleural effusion (n=2) and persistent air leak (n=1). Major complications included cervical anastomotic leak (n=1), and delayed gastric emptying requiring pyloroplasty (n=1). There was no perioperative mortality.
Conclusions:
This preliminary experience suggests that minimally invasive esophagectomy is safe and feasible in centers with experience in advanced minimally invasive surgical procedures. Further studies are necessary to determine advantages over open esophagectomy.
Keywords: Esophagectomy, Minimally invasive surgery, Laparoscopic surgery, Video-assisted thoracoscopic surgery
INTRODUCTION
Advances in minimally invasive technology and surgical techniques have allowed more complex procedures to be performed including laparoscopic anti-reflux surgery, laparoscopic myotomy for achalasia, laparoscopic and thoracoscopic staging for esophageal cancer, 1,2 videoassisted thoracoscopic (VATS) lobectomy,3,4 VATS Belsey fundoplication,5,6 VATS lung reduction surgery for emphysema,7 and many others. Esophagectomy for benign and malignant disease is a complex and challenging surgical procedure that can be associated with significant morbidity and mortality.8 In an effort to decrease the morbidity associated with esophageal resection, surgeons are beginning to apply minimally invasive techniques to facilitate esophagectomy. The aim of this report is to describe our initial experience using minimally invasive surgical approaches to esophagectomy.
METHODS
From June 1996 to May 1997, eight patients underwent esophagectomy at the University of Pittsburgh Medical Center using minimally invasive techniques alone or in combination with mini-laparotomy or mini-thoracotomy. The decision to perform all or part of the procedure using minimally invasive techniques was based on body habitus, prior surgery, tumor size and location, and surgeon's preference. Addition of an open procedure was applied if there were technical difficulties precluding completion of the operation by minimally invasive techniques. Data were collected for demographics, operative time, complications, length of hospital stay and the number of conversions to an open procedure.
SURGICAL TECHNIQUE
1. Laparoscopic transhiatal esophagectomy:
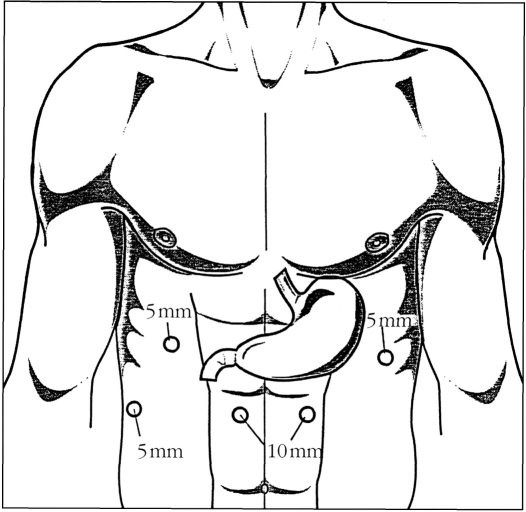
The patient were placed in a supine position with the head turned slightly to the right. The surgeon stood on the patient's right side with the assistant on the left. Five abdominal ports were placed (Figure 1) with the patient positioned in a steep reverse Trendelenburg position. Esophageal hiatus was exposed by retracting the left lobe of the liver upward using the Medi-flex (Velmed, Wexford, PA) self-retaining retractor system. The gastrohepatic ligament was divided exposing the right crus of the diaphragm. The retroesophageal space was developed and the esophagus was mobilized circumferentially. The stomach was mobilized with division of short gastric vessels using the ultrasonic shears (U.S. Surgical Corporation, Norwalk, Connecticut). Gastrocolic omentum was divided with preservation of the right gastroepiploic arcade. The stomach was retracted superiorly and the left gastric vessels were identified and divided using the Endo-GIA stapler (U.S. Surgical Corporation). A pyloromyotomy was performed using the Endoshear (U.S. Surgical Corporation) and electrocautery. Gastroduodenoscopy with insufflation was used in some cases to confirm the completion of the myotomy and absence of leaks.
Figure 1.
Abdominal trocar position for laparoscopic transhiatal esophagectomy.
The periesophageal dissection was accomplished under direct visualization by retracting downward on the stomach and dividing the aortoesophageal branches. This dissection was carried up to the level of the left main stem bronchus. The entire dissection was performed using the ultrasonic shears (U. S. Surgical Corporation). The esophagus with a cuff of normal gastric cardia was divided from the remaining stomach using the 4.5 mm Endoscopic stapler (Ethicon, Cincinnati, Ohio). The distal esophagus was sutured to the gastric conduit using 2-0 Surgitek Endostitch (U.S. Surgical Corporation). A 5 cm horizontal neck incision was used to mobilize the cervical esophagus. Mediastinoscope was inserted along the periesophageal plane to facilitate circumferential dissection of the upper esophagus. Once the entire esophagus was completely mobilized, it was removed through the neck incision by applying traction to the attached gastric conduit transhiatally up to the cervical incision. An esophagogastric anastomosis was performed using a 25 mm EEA stapler (U.S. Surgical Corporation) through a small gastrotomy. A nasogastric tube was directed through the anastomosis and placed down into the distal stomach. A laparoscopic feeding jejunostomy tube was performed by attaching a loop of proximal jejunum to the anterior abdominal wall. A Seldinger technique was used to place the feeding tube into the efferent limb of the jejunum. Catheter position was checked using intraoperative fluoroscopy.
2. Thoracoscopic and laparoscopic esophagectomy:
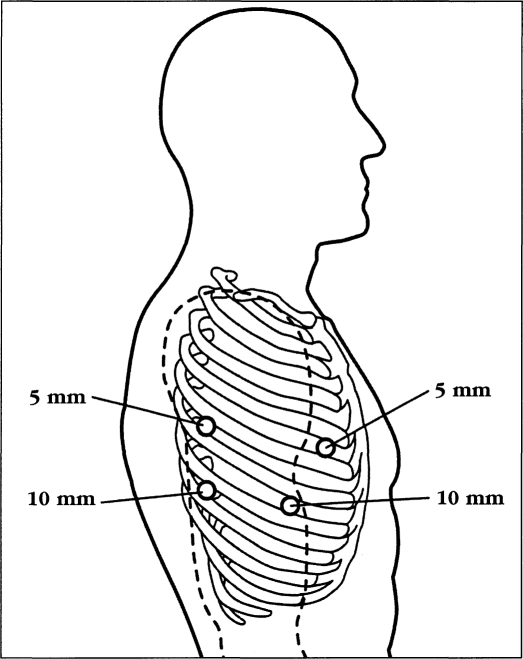
The patient was intubated with a double lumen tube for single lung ventilation and was positioned in the left lateral decubitus position with the right lung collapsed. Four thoracic trocars were introduced (Figure 2). Four 10 mm ports were used initially, but currently we use two 10 mm and two 5 mm ports. The camera port was placed at the seventh intercostal space, mid-axillary line. Another 10 mm port was placed at the eighth or ninth intercostal space 2 cm behind the posterior axillary line for the ultrasonic shears. One 5 mm port was placed posterior to the tip of the scapula and one at the fourth intercostal space at the anterior axillary line for placement of retracting instruments. A 0-Surgitek Endostitch (U.S. Surgical Corporation) was placed on the central tendon area of the diaphragm and brought out of the thorax inferiorly to facilitate downward retraction of the diaphragm and promote exposure of the distal esophagus. The mediastinal pleural was divided and the esophagus was circumferentially mobilized. A penrose drain was placed around the esophagus to facilitate retraction. The azygos vein was mobilized and divided using the Endo-GIA stapler with vascular cartridge (U.S. Surgical Corporation). The esophagus was mobilized from the diaphragmatic reflection up to the thoracic inlet. Periesophageal lymph nodes were sampled intraoperatively or mobilized to remain attached to the resected specimen. A 28 F chest tube was inserted through the camera port for postoperative drainage. The patient was then turned to the supine position. Laparoscopic gastric mobilization with cervical anastomosis was accomplished as described above.
Figure 2.
Thoracic trocar position on the right chest for thoracoscopic and laparoscopic esophagectomy.
RESULTS
Minimally invasive esophagectomy was performed on 8 patients (5 males, 3 females). Indications for esophagectomy included stage I carcinoma in 5 patients; palliative resection for dysphagia in 1 patient; Barrett's esophagus with high grade dysplasia in 1 patient; and 1 patient with end stage achalasia. Patients with carcinoma did not receive prior chemotherapy or radiation therapy. The mean age was 68 years with a range of 54-82 years (Table 1). Palliative resection was performed in one patient with good performance status and single metastatic lesion on the left lobe of the liver, which was resected by wedge excision at the time of esophagectomy. Two patients had prior esophageal surgery (hiatal hernia repair in one and a Collis-Nissen hiatal hernia repair in another).
Table 1.
Patient's demographics and diagnosis.
| Patients | Sex | Age | Diagnosis | Pathologic stage |
|---|---|---|---|---|
| 1 | M | 75 | Carcinoma | I |
| 2 | F | 72 | Achalasia | |
| 3 | M | 68 | Carcinoma | IIB |
| 4 | F | 75 | Carcinoma | IV |
| 5 | M | 65 | Carcinoma | IIA |
| 6 | M | 54 | Carcinoma | I |
| 7 | M | 83 | Carcinoma | I |
| 8 | F | 55 | Barrett's |
Minimally invasive approach to esophagectomy included total laparoscopic transhiatal esophagectomy in 4 patients; thoracoscopic combined with laparoscopic esophagectomy in 1; and laparoscopic gastric mobilization with right muscle-sparing mini-thoracotomy in 3 patients (Table 2). Conversion to mini-laparotomy was necessary to complete esophageal dissection in 1 patient and to facilitate gastric pull-up in another. Pyloromyotomy was performed on 4 patients and a pyloroplasty on 1 patient. A laparoscopic jejunostomy tube was placed in 3 patients. The mean operative time was 460 minutes (range of 267-570 minutes). The mean intensive care stay was 1.9 days (range of 0-7 days). The mean hospital stay was 13.8 days (range of 4-45 days). Minor complications included atrial fibrillation (n=1), pleural effusion (n=2) and persistent air leaks (n=1). Major complications included cervical anastomotic leak in 1 patient, and delayed gastric emptying requiring pyloroplasty in 1 patient without a pyloromyotomy at the original operation. The 30-day mortality was zero. The final pathology was stage I carcinoma in 3 patients (2 adenocarcinoma, 1 squamous cell); stage II in 2 patients (2 adenocarcinoma); stage IV in 1 patient for palliative resection (adenocarcinoma); Barrett's esophagus with high grade dysplasia in 1 patient; and 1 patient with benign end stage achalasia mega-esophagus.
Table 2.
Operative Procedure and Results.
| Patients | Operative Procedure | Conversion* | Operative Time (h) | ICU Stay (days) | Hospital Stay (days) | Postoperative Complications |
|---|---|---|---|---|---|---|
| 1 | LM/RT | No | 8 1/2 | 2 | 19 | Pleural effusior persistent air leaks |
| 2 | LTE | Yes | 8 3/4 | 7 | 45 | Anastomotic leaks |
| 3 | LM/TE | No | 7 1/4 | 0 | 4 | |
| 4 | LTE | No | 8 1/4 | 1 | 7 | Pleural effusior |
| 5 | LM/RT | Yes | 8 | 0 | 5 | |
| 6 | LTE | No | 6 3/4 | 2 | 13 | Atrial arrhythmia |
| 7 | LM/RT | No | 9 | 2 | 14 | Delayed gastric emptying |
| 8 | LTE | No | 7 3/4 | 1 | 4 |
Conversion to mini-laparotomy.
LM/RT - Laparoscopic mobilization/right thoracotomy with thoracic anatomosis. LTE - Laparoscopic transhiatal esophagectomy with cervical anatomosis.
LM/TE - Laparoscopic mobilization and thoracoscopic esophagectomy with cervical anatomosis.
DISCUSSION
The incidence of esophagus carcinoma in the United States is increasing at an alarming rate.9 The prognosis remains poor, with an overall five-year survival rate of 5-10%. Surgical resection of the esophagus is associated with significant morbidity and a mortality rate ranging from 2% to 10%.8 In an effort to decrease the morbidity and mortality associated with esophagectomy, minimally invasive techniques are being applied to perform this complex procedure.
Multiple authors have reported using video-assisted thoracoscopy or laparoscopy to facilitate esophagectomy.10–16 Most reports utilized a standard laparotomy with thoracoscopic esophageal mobilization or laparoscopy to facilitate gastric mobilization combined with a mini-laparotomy to bluntly complete the esophageal dissection. Clear advantages of thoracoscopic esophageal mobilization over thoracotomy were not demonstrated in these studies. DePaula was the first to report a large series of 48 patients undergoing a total laparoscopic transhiatal esophagectomy.15 In 2 patients, conversion to open surgery was required and 2 others required thoracoscopic assistance. Swanstrom recently reported nine cases of laparoscopic total esophagectomy.16 There were no conversions to laparotomy. One patient required a right thoracoscopy with intrathoracic anastomosis due to poor viability of the gastric tube.
We have extensive experience in the management of benign esophageal disorders using laparoscopic and/or thoracoscopic techniques. In addition, we routinely perform laparoscopic and thoracoscopic staging in patients with esophageal carcinoma for placement into a neoadjuvant therapy protocol.2 This experience led to the application of these techniques to esophagectomy.17 The operative ports for laparoscopic transhiatal esophagectomy are similar to our port placement in performing a laparoscopic Nissen fundoplication with the addition of a cervical incision. The laparoscopic transhiatal periesophageal dissection can be performed under direct visualization with the ability to biopsy surrounding mediastinal lymph nodes. Limitations for this approach include the small working space through the esophageal hiatus and difficulty in dissection of the mid and upper third esophagus due to the length of our instrumentation. In three cases, we performed laparoscopic gastric mobilization with a right mini-thoracotomy and intrathoracic anastomosis secondary to incomplete laparoscopic transhiatal esophageal mobilization. Therefore, we recently added thoracoscopic esophageal mobilization in one case as the first step followed by the laparoscopic gastric mobilization with transhiatal gastric pull-up. Thoracoscopic approach improves our ability to perform a wider lymph node dissection in these patients and improves our ability to mobilize the mid and proximal third of the esophagus following division of azygos vein.
CONCLUSIONS
Minimally invasive esophagectomy is a technically feasible operation requiring advanced laparoscopic surgical skills. This procedure is promising but time consuming and requires a steep learning curve. Appropriate instrumentations including the ultrasonic shears, endoscopic stapler and liver retractor are necessary to perform this procedure. This small series, similar to those of DePaula and Swanstrom, confirm that esophagectomy can be safely performed by the total laparoscopic or thoracoscopic approach in selected patients. Advantage over open approaches will require larger studies and longer followup.
Footnotes
This study was supported in part by a grant from United States Surgical Corporation.
Abstract presented at the annual meeting of American College of Gastroenterology, Chicago, Illinois, USA, 3-4 November 1997.
References:
- 1. Krasna MJ, Reed CE, Jaklitsch MT, et al. Thoracoscopic staging of esophageal cancer: a prospective, multiinstitutional trial. Ann Thome Surg. 1995;60(5):1337–1340 [DOI] [PubMed] [Google Scholar]
- 2. Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thome Cardiovasc Surg. 1997;114:817–823 [DOI] [PubMed] [Google Scholar]
- 3. Luketich JD, Kassis ES, Landreneau, et al. A comparison of total video thoracoscopic lobectomy and standard thoracotomy for early stage non-small cell lung cancer (NSCLC). [abstract] Lung Cancer. 1997;18:98 [Google Scholar]
- 4. Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy video-assisted thoracic surgery versus muscle-sparing thoracotomy. J Thorac Cardiovasc Surg. 1995;109:997–1002 [DOI] [PubMed] [Google Scholar]
- 5. Yang HK, Del Guercio LRM, Steichen FM. Thoracoscopic Belsey-Mark IV fundoplication. Surg Rounds. 1995:277–291 [Google Scholar]
- 6. Nguyen NT, Schauer PR, Hutson W, et al. Preliminary results of thoracoscopic Belsey Mark IV antireflux procedure. Surg Laparosc Endosc. 1998;8(3):185–188 [PubMed] [Google Scholar]
- 7. Keenan RJ, Landreneau RJ, Sciurba EC, et al. Unilateral thoracoscopic surgical approach for diffuse emphysema. J Thome Cardiovasc Surg. 1996;111(2):308–315 [DOI] [PubMed] [Google Scholar]
- 8. Lee RB, Miller JI. Esophagectomy for cancer. Surg Clin North Am. 1997;77:1169–1196 [DOI] [PubMed] [Google Scholar]
- 9. Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287. [PubMed] [Google Scholar]
- 10. Dexter SPL, Martin IG, McMahon MJ. Radical thoracoscopic esophagectomy for cancer. Surg Endosc. 1996;10:147–151 [DOI] [PubMed] [Google Scholar]
- 11. Robertson GM, Lloyd DM, Wicks AC, Veitch PS. No obvious advantages for thoracoscopic two-stage oesophagectomy. Br J Surg. 1996;83:675–678 [DOI] [PubMed] [Google Scholar]
- 12. McAnena OJ, Rogers J, Williams NS. Right thoracoscopically assisted esophagectomy for cancer. Br J Surg. 1994;81:236–238 [DOI] [PubMed] [Google Scholar]
- 13. Law S, Fok M, Chu KM, Wong J. Thoracoscopic esophagectomy for esophageal cancer. Surgery. 1997;122:8–14 [DOI] [PubMed] [Google Scholar]
- 14. Jagot P, Sauvanet A, Berthoux L, Belghiti J. Laparoscopic mobilization of the stomach for esophageal replacement. Br J Surg. 1996;83:540–542 [DOI] [PubMed] [Google Scholar]
- 15. DePaula AL, Hashiba K, Ferreira EAB, et al. Transhiatal approach for esophagectomy. In Toouli J, Gossot D, Hunter JG. eds. Endosurgery. New York: Churchill Livingstone; 1996:293–299 [Google Scholar]
- 16. Swanstrom LL, Hansen P. Laparoscopic total esophagectomy. Arch Surg. 1997;132:943–949 [DOI] [PubMed] [Google Scholar]
- 17. Luketich J, Nguyen N, Schauer P. Laparoscopic transhiatal esophagectomy for Barrett's esophagus with high grade dysplasia. JSLS. 1998;2:75–77 [PMC free article] [PubMed] [Google Scholar]