Abstract
Objective:
Laparoscopic repair of incisional ventral hernias with ePTFE mesh continues to evolve, with variable reporting of surgical techniques and outcomes. This report of 34 cases discusses, with a literature review of laparoscopic incisional hernia repair, specific factors associated with three recurrences.
Methods:
Retrospective analysis and review of the literature.
Results:
Thirty-two patients (16 female, 16 male), under-went 34 laparoscopic repairs: average age–54 years (27-80), average weight–207 lbs (100-300). Nineteen patients (62%) were undergoing first time repairs, 38% were redo cases and 5 cases (14%) involved previous mesh. Operating times averaged 101 minutes (45-220), and average length of stay was 1.9 days (0.6 days excluding 5 patients who required readmission), with 13 patients (38%) being discharged same-day. Two patients developed cellulitis (6%) treated without patch removal. Two enterotomies occurred (6%) both requiring patch removal. Five patients required readmission (14%), and one patient died postoperative day 29 secondary to end-stage liver disease. Three recurrences developed (9%): one secondary to missed enterotomy with reoperation, patch removal and hernia recurrence; one due to omission of suspension suture fixation; and one recurrence developed in a section of the intact old previous incision that extended beyond the original patch. Follow up has averaged 20 months (4-36).
Conclusions:
The laparoscopic repair of ventral and incisional hernias utilizing transabdominal placement of ePTFE patch can achieve excellent results with low morbidity in comparison with open surgical approaches. In reviewing the experience of other investigators, adequate fixation of the mesh, extension to cover the entire previous incision and standardizing the placement interval of the sutures are critical to the success of the repair.
Keywords: Ventral hernia, Laparoscopy, Laparoscopic surgical procedures, Minimally invasive surgery
INTRODUCTION
Leblanc and Booth first reported the laparoscopic approach to the repair of incisional ventral hernias (LIVH) in 1993,1 and several series have now demonstrated the efficacy of minimally invasive surgery in ventral incisional hernia (IVH) repair. Follow-up data, though relatively brief, indicate a significantly shorter postoperative length of stay—1-2 days versus 5-7 days— as compared with the classic open procedure as championed by Stoppa2 and Wantz.3
However, comparisons between studies are very difficult due to differences in cases and in surgical technique. For example, the terms “ventral hernia” and “incisional hernia” are frequently used interchangeably: the recently published multicenter trial included true ventral incisional hernias along with primary ventral hernias, such as umbilical hernias.4 Likewise, there are significant variations in technique, such as degree of patch overlap, choice of patch material and patch placement and in the use of suspension sutures. How these factors specifically influence complication rates and overall outcomes is largely undetermined.
This report examines complications and outcomes in 34 consecutive LIVH repairs in 32 patients by a single surgeon (Koehler), in close consultation with a national expert involved in the multicenter trial (Voeller). A comparison with recent laparoscopic series demonstrates wide variations in case selections and surgical techniques being reported. The three recurrences reported herein are used to emphasize key steps in surgical technique that we feel will have an impact on the success of LIVH.
METHODS AND MATERIALS
Patients
This retrospective study covers the period from January 1996 through January 1999, during which time 34 LIVH repairs were performed in 32 patients. (Two recurrences that were repaired are included.) Table 1 lists the overall patient demographics. The average age was 54 years (range 27-80 years), and the average weight was 207 lbs (range 100-300 lbs).
Table 1.
Patient Characteristics (34 Cases).*
| Male/Female | 18/16 |
| Average Age | 54 yrs |
| Type of Hernia | |
| Upper Midline* | 9 |
| Midline | 3 |
| Lower Midline | 5 |
| Epigastric | 4 |
| Umbilical | 3 |
| Subcostal | 6 |
| Paramedian | 3 |
| Lower Transverse | 1 |
| Prior Repairs (Recurrent Incisional) | 13 (38%) |
| Prior Mesh | 5 (15%) |
| Emergency Cases | 2 (6%) |
| Mesh Size (cm)† | |
| 15 x 19 | 21 (62%) |
| 18 x 24 | 10 (29%) |
| 2 patches sewn together | 3 (9%) |
Includes 2 recurrences reoperate d upon in this series.
Patch overlapped defect(s) by 5 cm.
All patients had true incisional hernias. Thirteen patients had at least one previous repair (38%), and five patients had previous synthetic mesh placement (15%). The types of previous repairs are shown in Table 1.
Operative Technique
The technique used here is well described in the multi-center trial,4 and adhered to in this study, with the exception of one case resulting in a recurrence discussed below. A two-sided, 1 mm ePTFE mesh (GORE-TEXDualMesh-Biomaterial, W.L. Gore & Assoc., Inc., Flagstaff, AZ) was used in all cases. Patients were prepped with alcohol and ioban-impregnated self-adhesive drape. Bladder and gastric decompression was employed in all cases. A direct cutdown approach was utilized for initiating pneumoperitoneum, with a 12 mm camera port being placed as far away from the defect as possible, often the left upper quadrant. A 45-degree, 10 mm laparoscope was used, and two 5 mm reusable ports were used for dissection, placed on opposite sides of the defect. Rarely, a third 5 mm port was placed. A 5 mm, 30-degree laparoscope was often used to assist in visualization and dissection of adhesions.
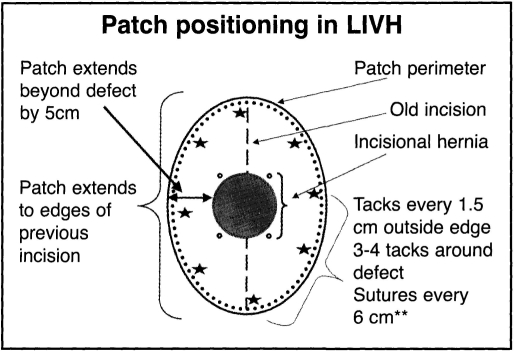
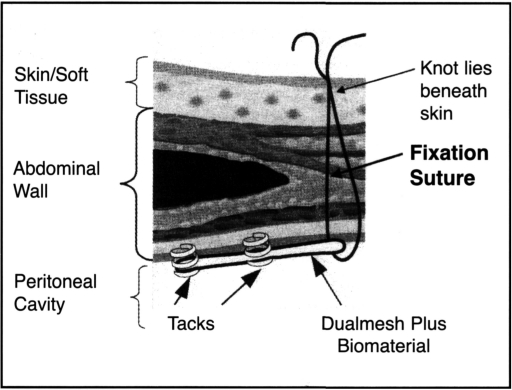
Adhesiolysis was always the most technically difficult part of each case. As little use of an energy source as possible was employed, and when energy was necessary high frequency harmonic coagulation shears, both 5 mm and 10 mm, were utilized (Ethicon Endo-Surgery, Inc., Cincinnati, OH). The entire previous incision was freed of adhesions, and the patch was selected to cover the defect by 5 cm in all dimensions while covering out to the edge of the prior incision (Figure 1). No attempt was made to reduce the hernia sac. Transfixing horizontal mattressed sutures were placed around the edges of the patch at 6 cm intervals, numbered on the patch surface and around the traced edge of the patch on the abdominal wall. A minimum of 6 sutures, and as many as 12 sutures, were used. The suture ends were cut. to 15 cm and clipped together. The patch was rolled sideto-side after placing the suture ends down in reverse order to facilitate retrieval, and the patch was brought into the abdomen through the removed 12 mm port by using one of the 5 mm port's graspers. After unrolling the patch, the individual sutures were brought up through 2 mm incisions using the Gore suture passer (W.L. Gore & Assoc., Flagstaff, AZ), angling the passer through the individual ends to create a mattressed tie across the abdominal wall. After tying the sutures, the peripheral edge of the patch was tacked into position with 5 mm tacks (Origin Medsystems, Menlo Park, CA) (Figure 2). Tacks were placed 1.5 cm apart (3 × the diameter of the tacker end), and, finally, several additional tacks were placed in an inner concentric pattern to close off dead space between the patch and abdominal wall. The entire procedure was carried out with 12-15 mm insufflation pressure, although pneumoperitoneum was deflated during the extracorporeal suture placement phase.
Figure 1.
Position of patch with respect to an incisional hernia; note overlap of defect, intervals for tacks and sutures, and patch reaching to edge of old incision.
Figure 2.
Transfixation sutures and tack placement through patch (image courtesy of W.L. Gore & Assoc.). Note: Suture should be mattressed through patch.
Antibiotics were used perioperatively (1 gm cefazolin intravenous), and postoperative pain control involved intravenous narcotics by patient controlled analgesia (PCA) as well as intravenous ketoralac, 30 mg loading followed by 10 mg IV q8hrs. Patients generally stayed in the hospital overnight for pain control and observation for occult intra-abdominal injury. Selected patients were discharged home the same day.
RESULTS
Results are summarized in Table 2. The average operating time was 101 minutes (range 45-220 minutes), varying in relationship to the degree of adhesiolysis required.
Table 2.
Operative Results.
| Operative Time (min)* | 101 (45-220) |
| Mesh Size (cm)† | |
| 15 x 19 | 21 (62%) |
| 18 x 24 | 10 (29%) |
| 2 patches sewn together | 3 (9%) |
| Length of stay (LOS) | |
| (includes complications/r,admissions) | 1.9 dys (0-29) |
| Excluding readmissions | 0.6 dys |
| Same day | 13 (38%) |
| Short Stay (24 hrs) | 12 (36%) |
| Readmit LOS (dys): 2,3,5,10,29 | |
| Follow-up | 20 mths (2-35) |
Skin incision to skin closure.
Patch overlapped defect(s) by 5 cm.
Mesh size used was 15 cm × 19 cm in 21 cases (62%), 18 cm × 24 cm in 10 cases (29%), and, in three cases, patches were sewn together to achieve coverage (9%). The average length of stay was 1.9 days—including all read-missions, with 13 patients (38%) being discharged the same day. All patients were discharged by postoperative day (POD) 2, with the exception of one case of delayed enterotomy recognized on postoperative day two, with return to the operating room and a prolonged postoperative course. Follow-up averages 20 months (range 2-36 months).
Complications are listed in Table 3 and are detailed below:
Table 3.
Complications.
| Cellulitis | 2 (6%) |
| Seroma | 2 (6%) |
| Enterotomy | 2 (6%) |
| Readmissions | 5 (14%) |
| 1-delayed enterotomy | |
| 1-cellulitis | |
| 1-SBO, unrelated to repair | |
| 2-Elective recurrence repairs | |
| Recurrences-Total | 3 (9%) |
| Primary recurrences | 2 (6%) |
| Secondary to patch removal for enterotomy | 1 (3%) |
| Postoperative death | 1 (3%) |
Cellulitis
Two primary infections occurred (6%), one treated out-patient and one inpatient without requiring mesh removal. Only two patients developed obvious seromas (6%), neither of which required drainage.
Readmissions
There were five readmissions (12%): two were elective for repair of recurrences; one was for cellulitis, which cleared with antibiotics; one was for delayed enterocutaneous fistula on POD 5; and one was for small bowel obstruction at two weeks postoperative, which was re-explored and found to be unrelated to the hernia repair.
Enterotomy
Two (6%) small bowel enterotomies occurred, neither of which were recognized at the time of surgery. One patient had done well and was discharged home on POD 2 and returned to the hospital on POD 5 with a small bowel enterocutaneous fistula. Reoperation revealed breakdown of the small bowel wall along the antimesenteric border in an area of extensive adhesiolysis. This required removal of the patch and resection of a small segment of the bowel. The patient recovered uneventfully but six months later has presented with a recurrence of her hernia. This represents one of the three recurrences. Although this appeared to be delayed breakdown of the bowel wall, it is listed as an enterotomy rather than as a “postoperative fistula.”
The second enterotomy occurred in a patient undergoing repair of a recurrence and, therefore, represents one of the two enterotomies, one of the three recurrences as well as the one postoperative death. This 62-year-old male had multiple previous attempts at repairing an upper midline incisional hernia, with previous polypropylene mesh insertion. His first LIVH repair in this series involved extensive adhesiolysis of small bowel from the polypropylene mesh and repair of the defect with ePTFE that measured 4 cm at the lower pole of the incision. The 15 × 19 cm patch overlapped the defect well but did not reach to the intact upper pole of the old incision. Fifteen months later, the segment of the original incision above the ePTFE patch had developed a “new” hernia, and was symptomatic. At repeat LIVH, there were loose adhesions to the upper aspect of the ePTFE patch, while dense adhesions were again encountered in the old polypropylene failed segment. A 15 × 19 cm ePTFE patch overlapped the entire defect. The post-operative recovery was eventful for an ileus POD 1, but the patient developed abrupt signs of peritonitis on the morning of POD 2. Return to the operating room revealed a 5 mm small bowel perforation, which was repaired, and the new ePTFE patch was removed. The original patch of 15-months duration was well-incorporated into tissues and left in place. The patient had return of bowel function by POD 4 and subsequently had no signs of systemic sepsis, but on POD 10 there was evidence of hepatic failure and what appeared to be acute hepatitis superimposed on chronic hepatitis and underlying occult cirrhosis. The patient went into complete hepatic failure and died POD 29.
Recurrences
Three patients (9%) developed recurrences, one related to mesh removal following delayed enterotomy discussed above, the second involving the case discussed above related to the defect appearing just above the original ePTFE patch placed 15 months previously: that area of the incision was entirely intact at the original LIVH, and the patch had overlapped the herniated portion of the incision by at least 6 cm.
The third recurrence developed in a 260 lb female under-going her second incisional hernia repair of an upper midline incision used originally for a gastric bypass procedure. Previous polypropylene mesh insertion had been placed, and LIVH revealed a 3 cm defect with extensive adhesions to the polypropylene. A 15 × 19 cm patch was placed and allowed such ample overlap that no suspension sutures were used—relying on two concentric rows of closely spaced 5 mm tacks. The patient was discharged within 24 hours and did well until 6 months later when an obvious recurrence had developed. At repeat LIVH, a few loose adhesions were noted to the ePTFE, although dense pinpoint adhesions were noted to exposed tack surfaces. The tacks had pulled out in many locations, with the mesh completely disrupted into the hernia defect. An 18 × 24 cm ePTFE DualMesh patch was placed with suspension sutures every 6 cm, and the patient was discharged POD 2. She is without recurrence at 22 months.
DISCUSSION
The repair of incisional ventral wall hernias (IVH) has been a challenging problem for the general surgeon. Substantially different techniques have been described in standard textbooks.5–7 The sometimes bewildering array of repair options, along with the frequent comorbidities in these patients, have led to often suboptimal results. Failure rates of 40-60% have been reported,8–11 and a 10-15% recurrence rate is considered very acceptable. Many authors have promoted the use of synthetic patch reinforcement of IVHs.3,5,11,12 Following Booth's report of LIVH in five cases,1 several more descriptions of LIVH appeared, culminating in the recent multicenter trial report of some 144 cases by nine authors.4
Unfortunately, comparing reported results is difficult and potentially misleading due to significant variations in terminology, patient selection and the operative technique employed.
Terminology and Case Selection
A close examination of recent series reveals that many of these studies have often included in their description of “ventral wall hernias” a significant percentage of primary umbilical and epigastric hernias (Table 4). In fact, 38% of the multicenter trial patients had primary umbilical and epigastric hernias. These primary “ventral wall” hernias may in fact be excellent first cases for the surgeon learning LIVH, provided the indications for patch closure are appropriate. However, we feel it is somewhat inappropriate to include these cases in the reporting of true IVH repairs. A patient with a primary ventral wall hernia can be expected to have far fewer adhesions, particularly versus an IVH where previous mesh closure has been attempted. Consequently, intraoperative complications such as enterotomy will be far fewer. Likewise, one might expect lower recurrence rates when repairing a primary umbilical hernia versus a recurrent incisional hernia with previous mesh placement. When comparing recurrence rates (Table 5), the multicenter trial reports 5% of 144 cases.4 If, however, these are looked at against only incisional hernia repairs in their series (92), the recurrence rate climbs to 8.5%. Costanza's 6% recurrence rate is notable given that these were all recurrent incisional hernias (RIVH).13 We have employed the term “incisional ventral hernia (IVH)” separately from “ventral hernia,” and have also distinguished recurrent incisional ventral hernias (RIVH). We believe it would be extremely useful to have consistency in the reporting of specific case types—for example, IVH, RIVH, VH—with respect to operative technique chosen, complications rates and follow-up.
Table 4.
Incisional versus “Ventral” Hernias in Selected Laparoscopic Series.
| Authors | Total Cases | IVH (% of total) | VH | RIVH |
|---|---|---|---|---|
| Koehler, 1998 | 34 | 34 (100%) | 0 | 13 (38%) |
| Ramshaw,24 1998 | 49 | ? unclear | ? | 25 (50%) |
| Toy,4 1998 | 144 | 92 (63%) | 52 (37%); 23 UH (15%) | 38 (26%) |
| Franklin,39 1998 | 176 | 112 (63%) | 64 (37%); 62 UH (35%) | 62 (35%) |
| Costanza,13 1998 | 16 | 16 (100%) | 0 | 16 (100%) |
| Tsimoyiannis,40 | 11 | 11 (100%) | 0 | 0 |
| Chari,26 1998 | 14 | ? unclear | ? | ? |
| Demaria,15 1998* | 21 | ? unclear | ? | 52% |
| Vargish,23 1998 | 45 | 45 (100%) | 0 | ? |
| Park,14 1998* | 56 | 56 (100%) | 0 | 16 (28%) |
| Holzman,22 1997* | 21 | ? unclear | ? | 8 (38%) |
| Park,18 1996 | 30 | 30 (100%) | 0 | ? |
IVH=Incision Ventral Hernia; VH=Primary Ventral Hernia; RIVH=Recurrent Incisional Ventral Hernia.
Studies comparing open IVH repair to LIVH repair; numbers indicate laparoscopic cohorts only.
Table 5.
Incisional versus “Ventral” Hernias in Selected Laparoscopic Series. Recurrences and Follow-Up.
| Authors | Total Cases | Incisional Hernias (% of total) | % Total | Recurrences % True incisional hernia | Follow-up-months |
|---|---|---|---|---|---|
| Koehler, 1998 | 34 | 34 (100%) | 3 (9%) | * | 20 |
| Ramshaw,24 1998 | 49 | ? unclear | 1 (2%) | ?, * | 22 |
| Toy,4 1998 | 144 | 92 (63%) | 8 (5%) | (8.5%) | 7 |
| Franklin,39 1998 | 176 | 112 (63%) | 2 (1%) | (1%) | 30 |
| Costanza,13 1998 | 16 | 16 (100%) | 1 (6%) | 18† | |
| Tsimoyiannis,40 | 11 | 11 (100%) | 0 | 15 | |
| Chari,26 1998 | 14 | ? unclear | 4 (28%) | ? | 6 |
| Demaria,15 1998 | 21 | ? unclear | 0 | ? | 11 |
| Vargish,23 1998 | 45 | 45 (100%) | 3 (6%) | ? | |
| Park,14 1998 | 56 | 56 (100%) | 6/45 (13%) | ‡ | 24 |
| Holzman,22 1997 | 21 | ? unclear | 2/20? (10%) | ? | 20 |
| Park,18 1996 | 30 | 30 (100%) | 1 (3%) | 8 |
Authors sited omission of suture fixation in recurrence cases.
All cases were RIVH.
45/56 cases involved in follow-up data; 6/56 total cases would yield a 10% recurrence rate.
Operative Technique
As with the open repair, there is still significant variation in patch material selection, in the description of patch fixation to the abdominal wall and in the amount of patch overlap to the defect (Table 6). The selection of patch material—principally being the choice between expanded polytetraflouroethylene (ePTFE) and polypropylene (PP)—is still a matter of debate. In fact, some reports include different closure techniques and even different patch materials among their cases.14,16
Table 6.
Laparoscopic Repair of Incisional and “Ventral” Hernias: Patch Material, Patch-to-Defect Overlap Recommended, Suture Fixation Recommended.
| Authors | Total Cases | Material | Patch Overlap to Defect | Suture Fixation |
|---|---|---|---|---|
| Koehler, 1998 | 34 | ePTFE(DM)* | 5 cm | Yes |
| Ramshaw,24 1998 | 49 | ePTFE(DM) | 3 cm | Yes |
| Toy,4 1998 | 144 | ePTFE(DM) | 3 cm | Yes |
| Franklin,39 1998 | 176 | polypropylene† | 3-5 cm | “Most Cases” |
| Costanza,13 1998 | 16 | ePTFE(DM) | 4 cm | Yes |
| Tsimoyiannis,40 | 11 | ePTFE(DM) | 2.5 cm | Yes |
| Chari,26 1998 | 14 | ePTFE(DM) | ? | ? |
| Demaria,15 1998 | 21 | ePTFE(DM) | ? | Yes |
| Vargish,23 1998 | 45 | polypropylene | 2.5-3 cm | No‡ |
| Park,14 1998 | 56 | both | 2.5 cm | Yes |
| Holzman,22 1997 | 21 | polypropylenes§ | 4 cm | No |
| Park,18 1996 | 30 | both | 2 cm | Yes |
ePTFE mesh (GORE-TEX® DualMesh® Biomaterial, W.L. Gore & Assoc. Inc. Flagstaff, AZ).
“…omentum is stapled in place and serves as a barrier to separate the mesh from the bowel.”39
Written presentation states sutures used, but in discussion states that sutures no longer used.
“Attempts to cover the mesh with omentum were made when possible.”22
There are five aspects of technique which are reviewed here:
1) Selection of patch material, principally PP versus ePTFE:
Whereas a recent review of the literature,16 both animal and human, on synthetic materials in man failed to clearly demonstrate a prohibitive rate of adhesion formation to PP, there are many reports of severe adhesion formation to PP when used directly in contact with the abdominal viscera. Nagy has reported extreme adhesions when using PP in trauma cases, and the authors have “abandoned Marlex for temporary closure.–17 Gagner's report utilizing both PP and ePTFE suggested that the ePTFE had advantages over PP as being less adhesiogenic.14 Park commented in their series that they prefer ePTFE for LIVH, due to its “. . . being less easily infected than polypropylene, and producing fewer and less tenacious adhesions.”18 Christoforoni, in an animal model, noted significantly more adhesions to PP materials than to ePTFE.19 In the literature on open repairs, Salky commented on ePTFE as being less adhesiogenic with less infection than PP.12 Leblanc and Booth recently reported on far greater adhesions from PP placed intra-abdominally in an animal model as compared with ePTFE.20 Finally, a recent retrospective study by Leber et al found a much higher complication rate when PP was used in IVH versus ePTFE, including a 16% incidence of bowel fistula formation with PP versus 1% for ePTFE. They concluded that “polyester mesh should no longer be used in incisional hernia repair.”21
Even authors recommending the use of PP mesh state that attempts should be made to interface the omentum between the PP patch and the viscera. Wantz's review specifically states that “. . . the intraperitoneal prosthesis (Mersilene) must be prevented from touching the viscera.”3 In true incisional hernias, placing the PP patch in a preperitoneal position via a laparoscopic approach is virtually impossible. Holzman and Eubanks, in commenting on their use of PP mesh, stated that “ . . . a preperitoneal approach to incisional hernias is virtually prohibitive. Attempts to separate the peritoneum of the hernia sac are met with serious obstacles, . . . results in a large peritoneal defect, . . . (and) leaves exposed mesh.”22 Nevertheless, at the recent American College of Surgeons 1998 Clinical Congress, Vargish has reported on the continued use of PP intra-abdominally.23
We feel that attempting to dissect out the sac will lead to more bleeding, with the potential as well of creating a communication between the frequently thinned-out overlying skin and the patch. No attempt was made to reduce the hernia sac in this series, and postoperative seromas occurred in 6% of patients. None required drainage, and all resolved over a period of 3-6 weeks.
In our series, three patients with ePTFE DualMesh were reoperated upon at 2 weeks, 6 months and 15 months. Adhesions of bowel to the ePTFE DualMesh were seen but were relatively tenuous and easily lysed. However, exposed titanium tack surfaces appeared to create particularly dense adhesions. In comparison, the five patients in our series with previous PP mesh were all found to have extremely dense adhesions involving the antimesenteric small bowel surface. In these cases, there is relative ischemia to the bowel wall when extensive adhesiolysis is performed, which may have accounted for reports of “delayed” enterotomy as seen in our series and also recently reported by others (see below).24,25
Due to the extreme adhesions between PP mesh and intra-abdominal contents that we and others have experienced in LIVH surgery, and considering that placement of mesh in a preperitoneal position in these cases is not possible, we feel that PP is not an acceptable material for LIVH repair—given the advantages of ePTFE with regards to adhesion formation. With respect to adhesion formation to the titanium tacks, it should be noted that staples as well as tacks are equally adhesiogenic, and the 5 mm tack, we believe, offers a superior holding ability with respect to the ePTFE mesh in the ventral abdominal wall.
2) Fixation of the patch to the abdominal wall:
Stoppa's classic thesis on recurrent complex groin hernias recom-mended lateral fixation of the large patch to prevent lateral migration. Likewise, with respect to incisional hernias, he states, “. . . the prosthesis must be maintained by peripheral sutures transfixing the (abdominal) wall.”2 We suspect that the forces on the myopectineal orifice—with it's rigid aspects—have to be substantially different than those of the anterior ventral abdominal wall. In the latter case, there is no “backstop” to the mesh, as there is with the groin anatomy and the bony pelvis. Therefore, the shearing force on the patch, and the degree of mobility of the anterior abdominal wall musculature, both combine to make a far more dynamic relationship between patch and abdominal wall than that seen in the groin.
We feel, as do Henniford13 and Ramshaw,24 that substantial fixation of the DualMesh ePTFE with permanent transabdominal wall sutures is critical to the success of LIVH. One of the failures reported here was clearly due to disruption of the mesh away from the abdominal wall. A recent abstract at the Society of American Gastrointestinal Endoscopie Surgeons (SAGES) suggested a high recurrence rate without suture fixation, even in a short-term follow-up.26 This was also seen by Eubanks22 with the use of PP without sutures, and this phenomenon has been reported in the open literature with PP: Molloy in 1991 felt that their recurrences were due to detachment of the mesh without fixation,27 and McCarthy reported the same findings ten years earlier.28 The same phenomenon has been reported with ePTFE.29–31
3) Patch overlap:
Here again, there are subtle differences in reports (Table 6). Based upon Stoppa's principles of giant reinforcement of the visceral sac, it seems obvious that the larger the overlap the better. In this series, although our recurrence due to patch disruption had an overlap of 9 cm, the absence of suture fixation was felt to be the cause. We strive for at least a 5 cm overlap, but we also place at least 8 and often 10-12 sutures around the patch circumference, as does Henniford.13
One of our recurrences was due to the initial repair not reaching to cover the upper aspect of the previous incision, although the original patch overlapped the original defect by 6 cm. We now strive to cover all of the previous incision during LIVH, believing that any part of the old incision is suspect to future disruption.
4) Avoidance of enterotomy and early identification when encountered:
Lysis of adhesions is arguably the most challenging part of LIVH. This is particularly true if previous synthetic mesh repair has been attempted. Enterotomy has been well-described in both the LIVH literature (Table 7) as well as in the open IVH reports.
Table 7.
Laparoscopic Incisional and Ventral Hernia Repair: Enterotomy Reports.
| Authors | Total Cases | IH (% of total) | Previous Repair with Mesh | Enterotomy |
|---|---|---|---|---|
| Koehler, 1998 | 34 | 34 (100%) | 13 (39%)/5 (15%) | 2 (6%) |
| Ramshaw,24 1998 | 49 | ? unclear | 25 (50%)*/? | 1 (2%) |
| Toy,4 1998 | 144 | 92 (63%) | 38 (26%)*/? | 2 (1.5%)* |
| Franklin,39 1998 | 176 | 112 (63%) | 62 (35%)*/? | 0* |
| Costanza,13 1998 | 16 | 16 (100%) | 16 (100%)/? | 0 |
| Tsimoyiannis,40 | 11 | 11 (100%) | ? | 0 |
| Chari,26 1998 | 14 | ? unclear | ? | 2 (14%) |
| Demaria,15 1998 | 21 | ? unclear | (52%) | 0† |
| Vargish,23 1998 | 45 | 45 (100%) | ? | ? |
| Park,14 1998 | 56 | 56 (100%) | ? | 0‡ |
| Holzman,22 1997 | 21 | ? unclear | ? | 1(6%)§ |
| Park,18 1996 | 30 | 30 (100%) | 0 | 0 |
Percent of total cases, including non-incisional ventral hernias.
One colocutanious fistula, ? missed sealed off enterotomy.
Recently reported 2 enterotomies in 75 cases (2.5%), American Hernia Society, Las Vegas 1999.
18 cases involved mesh insertion, 1/18=6% 2/21 involved resuturing previous mesh disruption.
Read and Yoder reported 11 enterotomies in 206 cases,32 and Gagner has reported a 10% incidence of enterotomy in laparoscopic surgery for emergency small bowel obstructions.33 Certainly, approaching an emergency obstructed LIVH should be considered a higher-risk case for enterotomy.
Again, however, comparing series can be misleading. One has to consider the case selections in these series: how many true IVH repairs versus primary VH repairs; how many RVH repairs with previous mesh placement. Although some series have reported 0% enterotomies, these same series often have patients with unexplained sepsis (Gillion34) or with “major systemic complications” (McLanahan35). These may in fact represent occult enterotomies that self-sealed.
The two cases in the first author's (RHK) experience represented the 30th and 35th LIVHs. One patient who went home on POD 2 and developed a “fistula” on POD 5 represents, we believe, a delayed breakdown of a small bowel wall injury. Indeed, there are reports in the literature of “mesh erosion” through the bowel wall,12,15,21 without specific reference to the time from original surgery. It is quite likely that there was relative compromise of the bowel wall at the time of surgery, which subsequently broke down and presented later as a complete perforation in the form of a mesh-to-bowel “fistula.” In the cases here, adhesiolysis was performed with direct cutting in one case and using harmonic coagulation shears in the other. The enterotomies appeared to have been delayed breakdowns of the antimesenteric border in the area of adhesiolysis, as neither enterotomy was observed at the original surgery.
Two recent reports on monopolar electrosurgery highlight the problem of dissection of adhered bowel loops away from the abdominal wall.36,37 Although bipolar current and the use of harmonic coagulation shears may, in theory, obviate some of the disadvantages on monopolar cautery, it should be noted that any energy source is capable of full-thickness bowel wall injury. Ramshaw reported that one of two enterotomies in their 79 cases (2.5%) was occult and not immediately recognized.24 Park's series in 1998 showed no enterotomies18 in 56 cases, but recently their updated experience in 75 cases revealed 2 enterotomies (2.5%).25
Clearly, as experienced surgeons perform more difficult cases, enterotomy reports will likely be higher. The surgeon should always consider the possibility of an occult partial thickness injury converting itself into a full-thickness bowel perforation when encountering a patient who is deteriorating after an otherwise uneventful LIVH. We always council patients carefully about the risk of bowel injury in LIVH, particularly if there has been a previous repair involving mesh insertion.
5) Decision for patch removal due to enterotomy:
Although a difficult option to choose in the advent of an enterotomy during LIVH, the standard approach should be for patch removal and open primary repair. There may be situations where there is negligible small bowel spillage with an immediately identified enterotomy, where repair and patch placement is acceptable. However, the surgeon early in his or her LIVH experience would be best advised to abandon patch placement. Certainly, in the case of delayed enterotomy recognition, obstructed small bowel enterotomy, or colon injury, patch placement is prohibitive. Temudom et al advised this in their recent report on open IVH repair with ePTFE.31
It is significant as well that many recurrences are secondary to patch removal under either the above circumstances or secondary to primary patch infection (Table 8). One of our three recurrences was in a case of enterotomy-related patch removal, and this has been reported by Toy in the multicenter series, where 2 of 8 recurrences (25%) were due to patch removal for infection.4 Heniford reported that their one recurrence was related to patch removal for infection.13 Roth and Park's data in 75 cases had 7 recurrences (9%), 2 of which (28% of recurrences) were related to patch removal for infection.25 In Rams haw's series of now 79 cases, one mesh was removed when an occult enterotomy was recognized, with no reported recurrence in that patient at short-term follow-up.24
Table 8.
Incisional versus “Ventral” Hernias in Selected Laparoscopic Series: Primary Mesh Infection*
| Authors | Total Cases | Incisional Hernias (% of total) | Mesh Infection (primary)* |
|---|---|---|---|
| Koehler, 1998 | 34 | 34 (100%) | 2 (6%) |
| Ramshaw,24 1998 | 49 | ? unclear | 1 (2%) |
| Toy,4 1998 | 144 | 92 (63%) | 5 (3%)† |
| Franklin,39 1998 | 176 | 112 (63%) | 1 (0.5%)‡ |
| Costanza,13 1998 | 16 | 16(100%) | 2(12%)§ |
| Tsimoyiannis,40 | 11 | 11 (100%) | 0 (1 “trocar site” infection) |
| Chari,26 1998 | 14 | ? unclear | 0 (2 related to enterotomy) |
| Demaria,15 1998 | 21 | ? unclear | 1 (5%) |
| Vargish,23 1998 | 45 | 45 (100%) | ? |
| Park,14 1998 | 56 | 56 (100%) | 2 (3.5%) |
| Holzman,22 1997 | 21 | ? unclear | 1 (5%)-mesh removed |
| Park,18 1996 | 30 | 30 (100%) | 0 (1 “trocar site” infection) |
Exluding mesh removal secondary to enterotomy-related complications.
Percent of all cases including non-incisional ventral hernias; 2 required mesh removal and led to repair failure; therefore, 2/8 failures in this series were due to infected mesh removal.
Required mesh removal; also had 3 “early” trocar-site infections.
Series of recurrent complex incisional hernias; one “cellulitis,” one “mesh infection” requiring removal and resulted in the one recurrence in the series.
We council our patients preoperatively that in the event of a difficult adhesiolysis and enterotomy repair, there may be a need to abandon patch placement and that this may result in a higher chance of recurrence. We would offer a “second stage” patch placement if an enterotomy is encountered and repaired. This would have the advantage of keeping the total repair via a laparoscopic approach, obviating the increased morbidity of an open approach under circumstances where the patient could not benefit from patch insertion due to contamination from the enterotomy.
6) Associated chronic liver disease:
The one death in our series was directly related to previously unrecognized chronic liver disease, secondary to Hepatitis C and cirrhosis, regressing into acute fulminant viral hepatitis and liver failure. Two reports briefly mention an association of chronic liver disease and incisional hernias: Lamont and Ellis in 1988 found jaundice as an independent risk factor in 23% of their patients with incisional hernias,38 and Bauer noted undiagnosed liver dis-ease as a causative factor in one of three recurrences in 28 giant incisional hernia repairs.12
We have noted incidentally that in our series of 34 patients, 5 (15%) had chronic viral hepatitis. While cirrhosis with ascites has been a classic warning sign in pri-mary umbilical hernias, we are not aware of any reports specifically correlating chronic viral hepatitis with the development of incisional hernias. Given the significant incidence of chronic hepatitis infection in the general population, and the role of the liver in protein and collagen synthesis, it seems not unreasonable to postulate that there may be an association between occult chronic hepatitis and the development of incisional hernias.
CONCLUSIONS
Laparoscopic repair of incisional ventral hernias is a promising, and still new, technique in the approach to this challenging and common problem in general surgery. Whereas at least 11 series are now reported varying from 20 to 144 cases, significant differences in case reporting make comparing results potentially misleading. Some authors included a large percentage of primary umbilical and epigastric ventral hernias, while others have a large proportion of recurrent complex incisional hernias. Repair strategies differ with respect to synthetic material used (polypropylene versus ePTFE), degree of patch overlap and use of transabdominal fixation sutures.
In this series of 34 incisional ventral hernias with three recurrences (9%), and in reviewing the available series in detail, we feel the following points are critical to the success of the repair:
Avoid first cases where there has been previous mesh insertion, and council patients carefully about the possibility of bowel injury during dissection obligating the abandonment of patch insertion, with the possibility of a “second stage” mesh insertion.
Use minimal energy—none if possible—during adhesiolysis; repair any questionable serosal tears.
Allow at least a 5 cm patch overlap to the defect, and cover all of the previous incision—even if not involved in the hernia—whenever possible.
Use ample fixation sutures around the patch at 6 cm intervals.
Avoid the use of polypropylene directly in contact with the intra-abdominal contents due to the severity of adhesions encountered in our own experience, as well as in the experience of others.
Add an inner concentric ring of tacks against the patch to help close off dead space, which in turn appears to lessen postoperative seroma formation.
Have a very low threshold to return to the operating room to search for an occult enterotomy in the non-improving postoperative patient.
Consider the possibility of occult chronic liver disease in an incisional hernia patient, particularly if there appears to be no apparent reason—such as an associated wound infection or associated aneurysmal disease— for the development of the hernia.
Authors should standardize their reporting to ensure that LIVHs are reported separately from primary VHs and also to recognize the increased complications with RIVH repairs.
We feel that by following these guidelines, based upon our own experience and that of 11 other series reviewed herein, LIVH repair can achieve excellent postoperative results with an acceptable, and comparatively low, complication rate and a significantly shorter length of hospital stay.
Footnotes
This paper was presented at the 7th International Meeting of Laparoendoscopic Surgeons, SLS Annual Meeting, Endo Expo ‘98, Dec. 9-12, 1998 in San Diego, CA.
Contributor Information
Richard H. Koehler, Fellow of the American College of Surgeons (FACS).; Martha's Vineyard Hospital, Martha's Vineyard, MA. Clinical Associate in Surgery, Massachusetts General Hospital, Boston, MA.
Guy Voeller, Fellow of the American College of Surgeons (FACS).; Associate Professor of Surgery, University of Tennessee, Memphis, TN.
References:
- 1. LeBlanc KA, Booth WV. Laparoscopic repair of incisional hernias using expanded polytetrafluorethylene: preliminary findings. Surg Laparosc Endo. 1993;3:39–41 [PubMed] [Google Scholar]
- 2. Stoppa RE. The treatment of complicated groin and incisional hernias. World J Surg. 1989;13:545–554 [DOI] [PubMed] [Google Scholar]
- 3. Wantz G. Incisional hernioplasty with Mersilene. Surg Gyn Obst. 1991;172:129. [PubMed] [Google Scholar]
- 4. Toy FK, Bailey RW, Carey S, et al. Prospective, multicenter study of laparoscopic ventral hernioplasty. Surg Endo. 1998;12:955–959 [DOI] [PubMed] [Google Scholar]
- 5. Condon RE. Incisional Hernia. In Nyhus LM, Condon RE, ed. Hernia (4th ed). Philadelphia: Lippincott Co; 1995:319–339 [Google Scholar]
- 6. Abrahamson J. Hernias. In Schwartz SI, Ellis H, ed. Maingot's Abdominal Operations (9th ed). Philadelphia: Appleton and Lang; 1989:273–296 [Google Scholar]
- 7. Santora TA, Roslyn JJ. Incisional hernia. In Rutkow IM, ed. Surgical Clinics of North America: Hernia Surgery. Philadelphia: WB Saunders & Co.; 1993:557–570 [DOI] [PubMed] [Google Scholar]
- 8. Gecim E, II, Kocak S, Ersoz S, et al. Recurrence after incisional hernia repair: results and risk factors. Surg Today. 1996;26:607–609 [DOI] [PubMed] [Google Scholar]
- 9. Hesselink VJ, Luijendijk RW, deWilt J, et al. An evaluation of risk factors in incisional hernia recurrence. Surg Gyn Obst. 1993;176:228–234 [PubMed] [Google Scholar]
- 10. Koller R, Miholic J, Jakl RJ. Repair of incisional hernias with ePTFE. Eur J Surg. 1997;163:261–266 [PubMed] [Google Scholar]
- 11. George CD, Ellis H. The results of incisional hernia repair: a twelve year review. Ann Roll Coll Surg Eng. 1986;68:185–187 [PMC free article] [PubMed] [Google Scholar]
- 12. Bauer JJ, Salky BA, Gelernt IM, Kreel I. Repair of large abdominal wall defects with ePTFE. Ann Surg. 1987;206:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costanza MJ, Henniford BT, Arca MJ, et al. Laparoscopic repair of recurrent ventral hernias. Am Surg. 1998;64:1–7 [PubMed] [Google Scholar]
- 14. Park A, Gagner M, Pomp A. Laparoscopic repair of large incisional hernias. Surg Lapar Endo. 1996;6:123–128 [PubMed] [Google Scholar]
- 15. Demaria EJ, Moss JM, Sugerman H. Laparoscopic intraperitoneal PTFE prosthetic path (LIPP) of ventral hernia (VH): prospective comparison to open prefascial polypropylene mesh (OPPM). Surg Endo. 1998;12(suppl):S10. [DOI] [PubMed] [Google Scholar]
- 16. Morris-Stiff H. The outcomes of nonabsorbable mesh. J Am Coll Surg. 1998;186:352–367 [DOI] [PubMed] [Google Scholar]
- 17. Nagy KK, Fildes JJ, Mahr C, et al. Experience with three prosthetic materials in temporary abdominal wall closure. Am Surg. 1996;62:331–335 [PubMed] [Google Scholar]
- 18. Park A, Birch DW, Lovrics P. Laparoscopic and open incisional hernia repair: a comparison study. Surgery. 1998;124:816–822 [DOI] [PubMed] [Google Scholar]
- 19. Christoforoni PM, Kim YB, Preys Z, et al. Adhesion formation after incisional hernia repair: a randomized porcine trial. Am Surg. 1996;62:935–938 [PubMed] [Google Scholar]
- 20. LeBlanc KA, Booth WV, Whitaker JM, Baker D. In vivo study of meshes implanted over the inguinal ring and external iliac vessels in uncastrated pigs. Surg Endo. 1998;12:247–251 [DOI] [PubMed] [Google Scholar]
- 21. Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133;378–382 [DOI] [PubMed] [Google Scholar]
- 22. Holzman MD, Purut CM, Reinigen K, et al. Laparoscopic Ventral & Incisional Hernioplasty. Surg Endo. 1997;11:32–35 [DOI] [PubMed] [Google Scholar]
- 23. Vargish T. Laparoscopic repair of ventral hernia. In American College of Surgeons, Postgraduate Course 7. 1998:36–37 [Google Scholar]
- 24. Ramshaw B. Data presented at the Society of Laparoendoscopic Surgeons, 7th International Meeting, San Diego, CA, December 9-12, 1998 [Google Scholar]
- 25. Park A, Roth S. Data presented at the American Hernia Society, 3rd Annual Meeting, Las Vegas, NV, February 23-25, 1999 [Google Scholar]
- 26. Chari R, Chari V, Eisenstat M. A case-controlled study of laparoscopic ventral hernia repair. Surg Endose. 1998;12(suppl):S09. [DOI] [PubMed] [Google Scholar]
- 27. Molloy RG, Moran KT, Walar-on RP, Brady MP, Kirwan O. Massive incisional hernia: abdominal wall replacement with Marlex mesh. Br J Surg. 1991;78:242–244 [DOI] [PubMed] [Google Scholar]
- 28. McCarthy JD, Twiest MW. Intraperitoneal polypropylene mesh support incisional herniorraphy. Am J Surg. 1981;142:707–711 [DOI] [PubMed] [Google Scholar]
- 29. Bellon JM, Contreras LA, Sabeter C, Bujan J. Pathologic and clinical aspects of repair of large incisional hernias after implant of PTFE prosthesis. World J Surg. 1997;21:402–406;disc 406–407 [DOI] [PubMed] [Google Scholar]
- 30. Monaghan RA, Meban S. ePTFE patch in the hernia repair: a review of clinical experience. Can J Surg. 1991;34:50–55 [PubMed] [Google Scholar]
- 31. Temudom T, Siadati M, Sarr MG. Repair of complex giant or recurrent ventral hernias by using tension-free intraparietal prosthetic mesh (Stoppa technique): lessons learned from our initial experience (fifty patients). Surgery. 1996;120:738–743;disc 743 [DOI] [PubMed] [Google Scholar]
- 32. Read RC, Yoder G. Recent trends in the management of incisional hernias. Arch Surg, 1989;124:485–488 [DOI] [PubMed] [Google Scholar]
- 33. Breton G, Pomp A, Gagner M. Complications related to laproscopic surgery for intestinal adhesions. Surg Endo. 1996;10:(suppl):S-155 [Google Scholar]
- 34. Gillion JF, Begin GF, Marecos C, Fourtanier G. ePTFE patches used in the intraperitoneal or extraperitoneal position for repair of incisional hernias of the anterolateral abdominal wall. Am J Surg. 1997;174:16–19 [DOI] [PubMed] [Google Scholar]
- 35. McLanahan D, King LT, Weems C, Novotney M, Gibson K. Retrorectus prosthetic mesh repair of midline abdominal hernia. Am J Surg. 1997;173:445–449 [DOI] [PubMed] [Google Scholar]
- 36. Brill AI, Feste MD, Hamilton TL. Patient safety during laparoscopic monopolar clectrosurgery-principles and guidelines. J Soc Laparoendo Surg. 1998;2:221–225 [PMC free article] [PubMed] [Google Scholar]
- 37. Vancaille TG. Active electrode monitoring; how to prevent unintentional thermal injury associated with monopolar electro-surgery at laparoscopy. Surg Endo. 1998;12:1009–1012 [DOI] [PubMed] [Google Scholar]
- 38. Lamont PM, Ellis H. Incisional hernia in re-opened abdominal incisions: an overlooked risk factor. Br J Surg. 1988;75:374–376 [DOI] [PubMed] [Google Scholar]
- 39. Franklin ME, Dorman JP, Glass JL, et al. Laparoscopic ventral incisional hernia repair. Surg Laparosc Endosc. 1998;8:294–299 [PubMed] [Google Scholar]
- 40. Tsimoyiannis EC, Tassis A, Glantzounis G, Jabarin M, et al. Laparoscopic intraperitoneal onlay mesh repair of incisional hernia. Surg Laparosc Endosc. 1998;8:360–362 [PubMed] [Google Scholar]