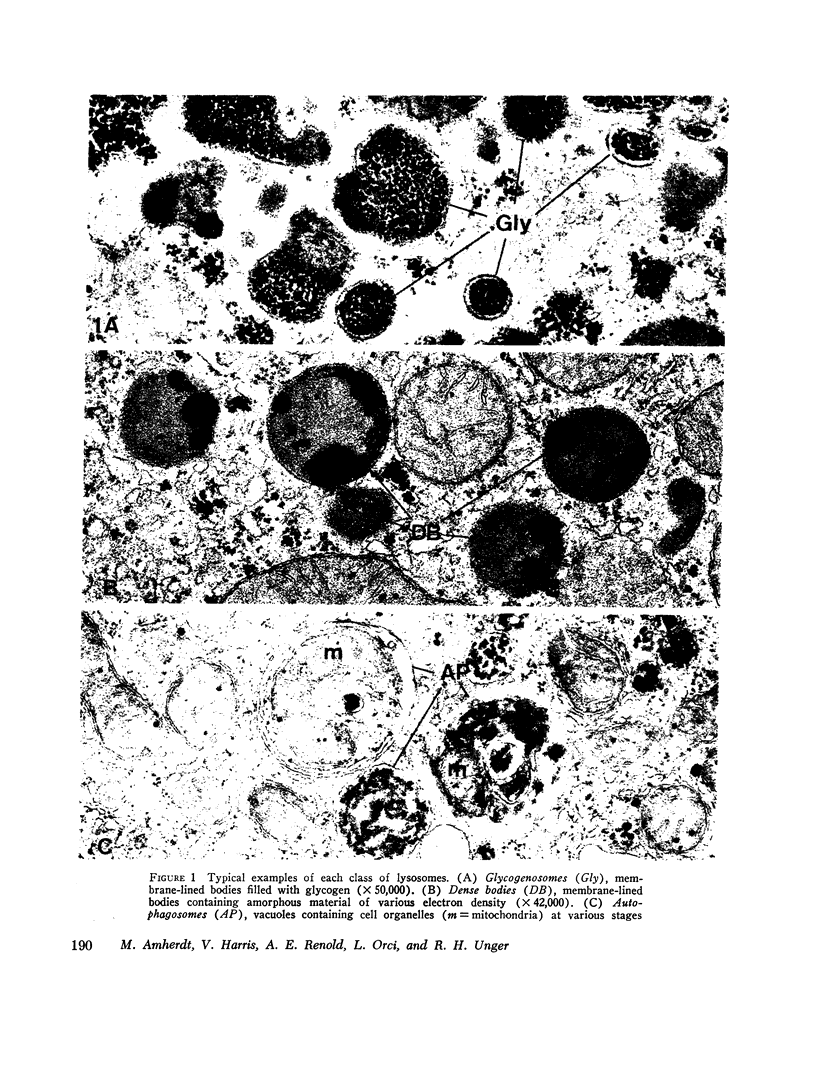
Abstract
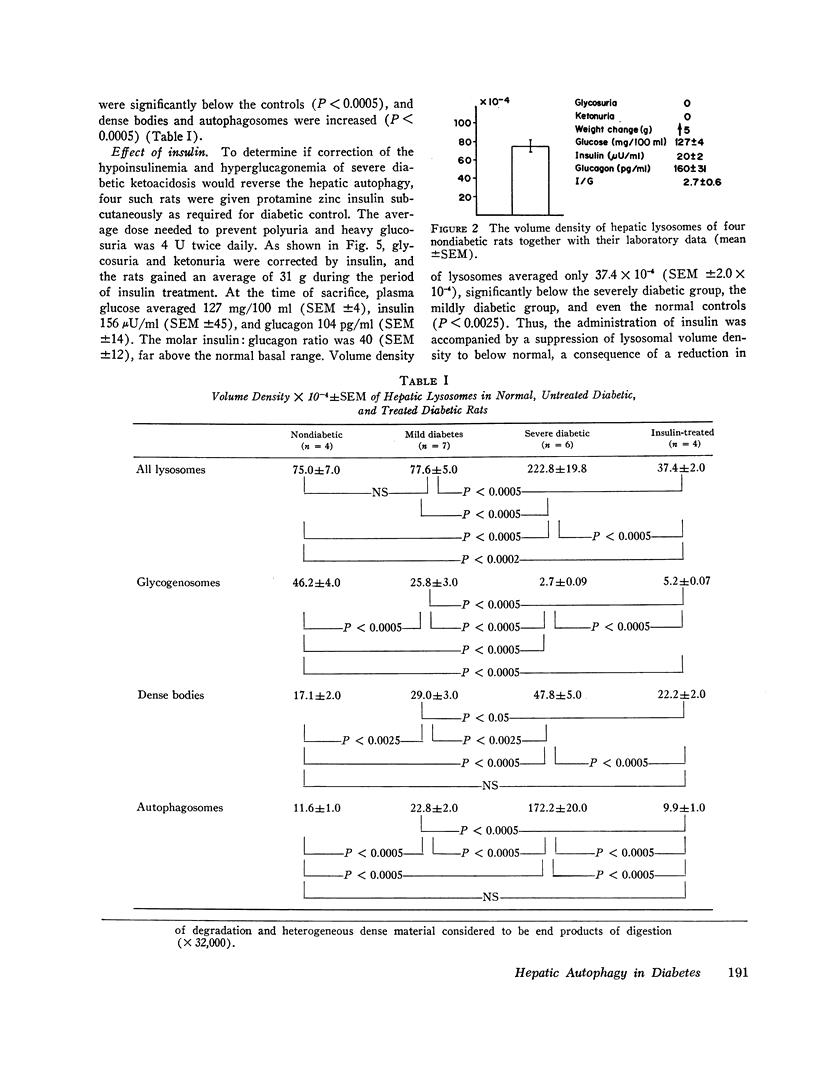
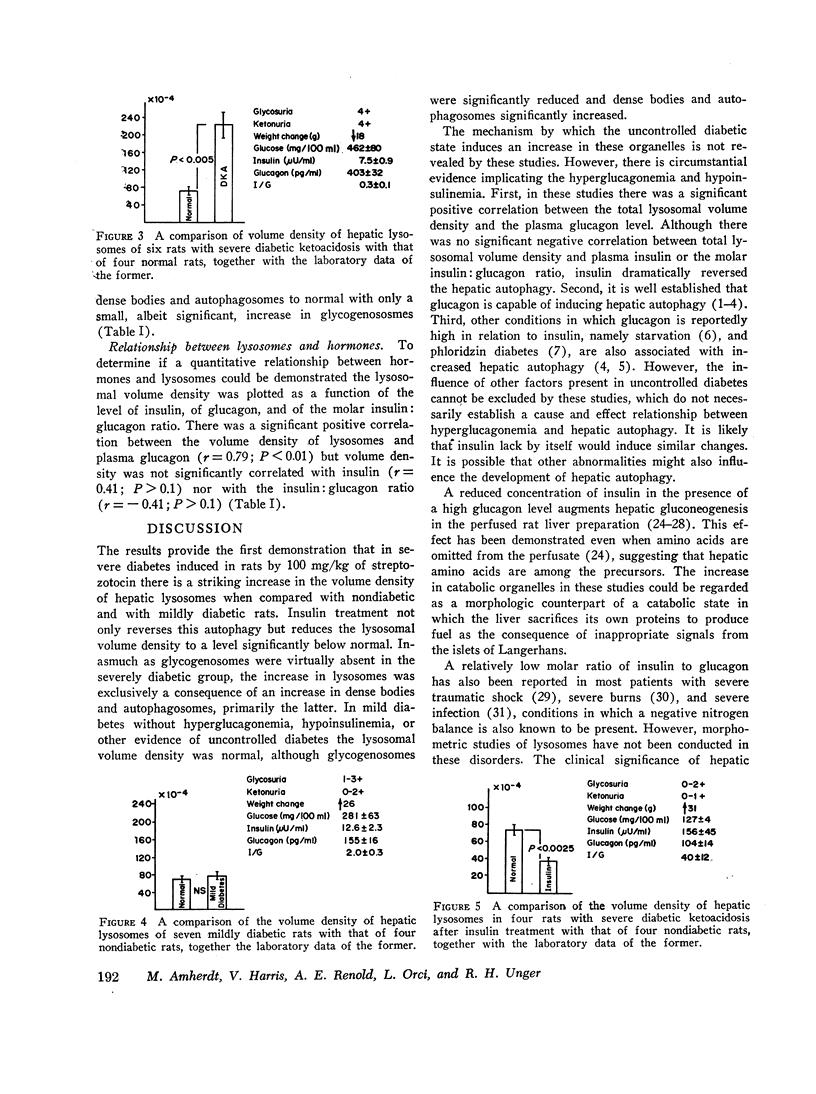
Exogenous glucagon is known to increase hepatic lysosomes, but the relationships between endogenous glucagon and insulin levels and hepatic lysosomes have not been examined. To determine if the hormones of the pancreatic islets influence the development of these organelles glycogenosomes, dense bodies, and autophagosomes were morphometrically quantitated in normal rats, in rats with mild streptozotocin diabetes with normal hormone levels, and in rats with severe streptozotocin diabetes with hyperglucagonemia, hypo-insulinemia, and clinical evidence of uncontrolled diabetes and ketoacidosis. In the latter volume density of lysosomes averaged 222.8×10-4 (SEM ±19.8×10-4), significantly above the control value of 75×10-4 (SEM ±7.0×10-4) (P<0.0005); glycogenosomes were absent in the diabetics, the increase being largely the result of increased autophagosomes. Insulin treatment corrected the hyperglucagonemia, hypoinsulinemia, and other manifestations of uncontrolled diabetes and reduced the volume density of lysosomes to 37.4×10-4 (SEM ±2.0×10-4), significantly below both the untreated diabetic rats and the nondiabetic controls (P<0.0025). In mild streptozotocin diabetes, in which hyperglucagonemia, hypoinsulinemia, and other evidence of uncontrolled diabetes were absent, lysosomes averaged 77.6×10-4 (SEM ±5.5×10-4), not different from the controls. A statistically significant correlation between all measurements of lysosomal volume density and plasma glucagon was observed (r=0.79; P<0.001). It is concluded that uncontrolled streptozotocin diabetes in rats is accompanied by hepatic autophagy which may be related to the increased plasma glucagon level and/or the decreased insulin and which is corrected by insulin therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968 Nov;53(5):687–733. [PMC free article] [PubMed] [Google Scholar]
- Assan R., Hautecouverture G., Guillemant S., Dauchy F., Protin P., Derot M. Evolution de paramètres hormonaux (glucagon, cortisol, hormone somatotrope) et énergétiques (glucose, acides gras, glycérol libre) dans dix acido-cétoses diabétiques graves traitées. Pathol Biol (Paris) 1969 Dec;17(23):1095–1105. [PubMed] [Google Scholar]
- Becker F. F., Cornwall C. C., Jr Phlorizin induced autophagocytosis during hepatocytic glycogenolysis. Exp Mol Pathol. 1971 Feb;14(1):103–109. doi: 10.1016/0014-4800(71)90056-6. [DOI] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Mortimore G. E. Influence of glucagon and 3', 5'-AMP on insulin responsiveness of the perfused rat liver. Am J Physiol. 1968 Sep;215(3):553–559. doi: 10.1152/ajplegacy.1968.215.3.553. [DOI] [PubMed] [Google Scholar]
- Guder W., Hepp K. D., Wieland O. The catabolic action of glucagon in rat liver. The influence of age, nutritional state and adrenal function on the effect of glucagon on lysosomal N-acetyl-beta, D-glucosaminidase. Biochim Biophys Acta. 1970 Dec 29;222(3):593–605. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsilambros N., Rahman Y. A., Hinz M., Fussgänger R., Schröder K. E., Straub K., Pfeiffer E. F. Action of streptozotocin on insulin and glucagon responses of rat islets. Horm Metab Res. 1970 Sep;2(5):268–270. doi: 10.1055/s-0028-1095056. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey A., Santeusanio F., Braaten J., Faloona G. R., Unger R. H. Pancreatic alpha-cell function in trauma. JAMA. 1974 Feb 18;227(7):757–761. [PubMed] [Google Scholar]
- MILLER L. L. Some direct actions of insulin, glucagon, and hydrocortisone on the isolated perfused rat liver. Recent Prog Horm Res. 1961;17:539–568. [PubMed] [Google Scholar]
- Mackrell D. J., Sokal J. E. Antagonism between the effects of insulin and glucagon on the isolated liver. Diabetes. 1969 Nov;18(11):724–732. doi: 10.2337/diab.18.11.724. [DOI] [PubMed] [Google Scholar]
- Menahan L. A., Wieland O. Interactions of glucagon and insulin on the metabolism of perfused livers from fasted rats. Eur J Biochem. 1969 May 1;9(1):55–62. doi: 10.1111/j.1432-1033.1969.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha D. M., Santeusanio F., Faloona G. R., Unger R. H. Abnormal pancreatic alpha-cell function in bacterial infections. N Engl J Med. 1973 Apr 5;288(14):700–703. doi: 10.1056/NEJM197304052881402. [DOI] [PubMed] [Google Scholar]
- Stäubli W., Hess R., Weibel E. R. Correlated morphometric and biochemical studies on the liver cell. II. Effects of phenobarbital on rat hepatocytes. J Cell Biol. 1969 Jul;42(1):92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBEL E. R., GOMEZ D. M. A principle for counting tissue structures on random sections. J Appl Physiol. 1962 Mar;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]