Abstract
Background:
The use of endoscopic saphenous vein harvesting (ESVH) for coronary artery bypass grafting (CABG) is growing. This study was done to investigate the extent of endothelial injury in ESVH compared with that of the standard open method (OSVH), and under various physical and chemical preservation factors.
Methods:
We endoscopically removed the saphenous vein from 45 consecutive patients undergoing saphenectomy for CABG together with a segment retrieved by the no-touch OSVH method. Vein samples from each group were divided into 8 subgroups of 5 samples each, and incubated in Plasma-Lyte solution with or without papaverine, at distending pressures of 100 or 300 mm Hg, and at either 4°C or 28°C, respectively. A ninth subgroup was preserved at room temperature without pressure or papaverine. The viability of cultured saphenous vein endothelial cells was assessed by counting the number of total cells and deriving the proportion of viable cells, following incubation for 72 hours.
Results:
The median proportion of viable cells (PVC) showed a slight decline over days 0 to 4 for both harvesting methods. No significant difference existed in the median PVC between the two techniques (day 0: 75%, 72%, P = 0.8; day 1: 66.7%, 66.7%, P = 0.9; day 2: 66.7%, 66.7%, P = 0.3; day 3: 65.3%, 66.7%, P = 0.16, respectively). The mean PVC compared across temperatures of 4°C, 28°C, and room temperature for the ESVH was highly significant, with the highest value being for room temperature (69.5%, 56.4%, 70.3%, respectively, P = 0.0003).
Results for the OSVH were not significant. The effect of distension pressure did not vary significantly for 0, 100, and 300 mm Hg for both techniques (70.3%, 63.2% and 63.4%, respectively, P = 0.46 for the ESVH; 66.5%, 68.4%, 67.4%, respectively, P = 0.94 for the OSVH). The addition of papaverine improved PCV slightly for the OSVH only (61.7%, 64.3%, respectively, P = 0.02), whereas that for the ESVH was not significant (67.3%, 72.5%, P = 0.12).
Conclusion:
The effect of ESVH on endothelial cell viability is comparable to that of the OSVH. Among the factors influencing endothelial viability during vein preparation, temperature had a major effect with lower temperatures in the range of 4°C to room temperature being the most favorable one. Mechanical distension and papaverine had unimportant or inconsistent roles. We recommend the ESVH as the procedure of choice for saphenous vein harvesting due to the lower postoperative morbidity, and the lower incubation temperature needed for its better influence on potential graft patency.
Keywords: Endothelial cell viability, Endoscopic saphenous vein harvesting, Open saphenous vein harvesting
INTRODUCTION
Meticulous preservation of the endothelial lining of vein grafts harvested during vascular operations is undoubtedly an important factor in determining patency rates following bypass procedures. Intact, confluent endothelium serves as an electrical, mechanical, and physiological barrier between flowing blood and the subendothelium. Destruction of the endothelial lining of the vein graft prior to graft implantation results in a more thrombogenic graft, which is essentially a collagenlined tube.1 Disruption of this barrier may also result in occlusive medial smooth muscle cell proliferation and intimal migration. In addition, the endothelium produces prostacyclin2 and exhibits plasminogen-dependent fibrinolytic activity.3,4
The integrity of the endothelial lining is affected by many factors, such as the technique of harvesting and preservation solution used. The optimal composition of the solution used for saphenous vein preparation in coronary bypass surgery (CABG) may influence the ultimate graft patency due to potential injurious effects of the solution constituents on the vein endothelium.5 In addition, environmental temperature, distending pressure, and the venodilator chemicals used in vein preparation play a major role in future vein conduit patency.6
With the introduction of endoscopic saphenous vein harvesting, complications are much less common compared with those that occur with the open technique, which include cellulitis, hematoma, seroma, edema, saphenous neuropathy/neuralgia, and ischemic sequelae, with lower morbidity for patients with risk factors, such as female sex, obesity, smoking, hypertension, diabetes mellitus, and peripheral vascular disease.1,7,8 Various modifications of this minimally invasive technique have been tried including the Richardson retractor, Mayo vein stripper with more than one small incision to minimize saphenous vein handling.9
The increased tendency of the graft to thrombose in the early postoperative period could be due to damage to the graft during the operation with injury and loss of the endothelium, damage through vein preparation, or technical pitfalls in the surgical anastomosis. The failure can be broadly categorized as either “early,” < one year posttransplantation, or “late,” > one year posttransplantation. The vast majority of failures occur in the “early” period, as shown by several studies in which implantation failure rates of CABG are 15% to 50% at one year.10–16 Further studies revealed that most of these failures occur within the first month after implantation.17 Late graft failures occur much less rapidly, approximately 2% to 6% per year.11,15,18 Many causes of late graft failures have been suggested including intimal hyperplasia, atheromatous disease, progression of underlying disease proximally and distally, compliance mismatch, chronic exposure to arterial pressures, rheologic factors, embolic phenomena, hyperlipidemia, and graft ischemia.17
Previous investigators have studied the effect on vein graft endothelium of various pressures, temperatures, and solutions used for dilatation of the graft.19–21 Gundy et al22 found that cold blood preserved the endothelium with minimal disruption in human saphenous grafts. They also noted that pressures more than 300 mm Hg used for dilatation of the graft caused endothelial damage.3
A lot of controversy still exists in spite of the use of papaverine in saphenous vein preparation. Rubens et al5 suggested that papaverine is a potent vasodilator; however, exposure to this compound might compromise long-term viability of graft endothelial cells. Vikrom et al23 confirmed the beneficial effect of papaverine in vein preparation by protecting the endothelium and smooth muscle cells in the intima and media by preventing leukocyte infiltration and medial fibrosis.
The purpose of this study was to compare the viability of endothelial cells isolated from saphenous veins that we harvested using two different techniques, the standard open and the endoscopic, under different temperatures and distending pressures. In addition, we evaluated the effect of papaverine in storage solutions commonly used for vein preservation in cardiovascular surgery.
MATERIALS AND METHOD
Vein Retrieval
The study was done at Maimonides Medical Center (MMC) from November 1998 to May 1999. We harvested saphenous veins from 45 patients prepared for CABG. In the standard technique of open (no-touch) vein harvesting, the greater saphenous vein is exposed and harvested under direct vision through a long continuous skin incision, with the patient's leg in a “frog-leg” position. Whereas in the endoscopic technique, a small 4-finger breadth incision is made posterior to the proximal margin of the patella, the greater saphenous vein is identified, dissected both cranially and distally under endoscopic visualization through the incision, and then stripped and retrieved. The standard instrumentation used in the procedure is commercially known as the Endo-Path (Ethicon Endo-Surgery, Inc, Cincinnati, OH). It comprises a subcutaneous dissector, retractor, and a modified vein stripper. In addition, standard endoscopic equipment, including a television monitor, light source, fiberoptic camera, and a 5-mm lens is used.
In our study, we sampled two vein segments, each by using one of the two harvesting techniques, from the same leg of each study patient. This was done by excising a 5-cm segment of the thigh portion of the saphenous vein, cranially dissected and retrieved endoscopically as the endoscopic sample. The sample was clipped distally for orientation of blood flow direction. At the same time, the vein segment remaining at the site of the incision, about 10 cm in length, was excised as the direct or open sample.
We harvested and handled the veins under sterile conditions according to the operating room protocol at MMC.
Vein Preparation
Vein samples were incubated in 10 mL Iscove's Modified Dullbecco's Medium (IMDM) with 200 μL penicillin-streptomycin during transport to the laboratory. In the laboratory, each vein sample was flushed and cannulated, injected with Plasma-Lyte, and the branches were ligated with 3.0 silk sutures.
Study Groups
Veins retrieved by each technique (OSVH or ESVH) were considered a separate group. Each group (45 samples) was subdivided into 9 subgroups, each containing 5 samples. The vein specimens in each subgroup were exposed to various temperatures, pressures, and solutions (Table 1).
Table 1.
The subgroups of the patients included in the study with temperature, pressure and papaverine as variables.
| Temperature | Pressure | Papavarine | |
|---|---|---|---|
| Group I | 28°C | 100 mm Hg | No |
| Group II | 4°C | 100 mm Hg | No |
| Group III | 28°C | 300 mm Hg | No |
| Group IV | 4°C | 300 mm Hg | No |
| Group V | 28°C | 100 mm Hg | Yes |
| Group VI | 4°C | 100 mm Hg | Yes |
| Group VII | 28°C | 300 mm Hg | Yes |
| Group VIII | 4°C | 300 mm Hg | Yes |
| Group IX | Control |
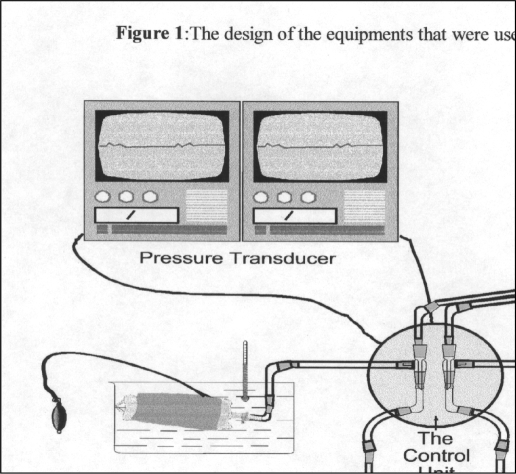
The details of vein preparation and handling in the lab in our department are illustrated in Figure 1.
Figure 1.
The design of the equipment that we used in our study with temperature 4°C or 28°C, pressure 100 mm Hg or 300 mm Hg and with or without papavarine.
Subgroup-1:
Vein segments were immersed in Plasma-Lyte solution without papaverine at 28°C and distended to 100 mm Hg with the same solution for 1 hour.
Subgroup-2:
The veins were immersed in Plasma-Lyte solution without papaverine at 4°C and distended to 100 mm Hg with the same solution for 1 hour.
Subgroup-3:
The veins were immersed in Plasma-Lyte solution without papaverine at 28°C and distended to 300 mm Hg with the same solution for 1 hour.
Subgroup-4:
The veins were immersed in Plasma-Lyte without papaverine at 4°C and then distended to 300 mm Hg with the same solution for 1 hour.
Subgroup-5:
The veins were immersed in Plasma-Lyte with papaverine at 28°C and then distended to 100 mm Hg with the same solution for 1 hour.
Subgroup-6:
The veins were immersed in Plasma-Lyte with papaverine at 4°C and then distended to 100 mm Hg with the same solution for 1 hour.
Subgroup-7:
The veins were immersed in Plasma-Lyte with papaverine at 28°C and then distended to 300 mm Hg with the same solution for 1 hour
Subgroup-8:
The veins were immersed in Plasma-Lyte with papaverine at 4°C and then distended to 300 mm Hg with the same solution for 1 hour.
Subgroup-9:
The veins in this subgroup were prepared as controls. Immediately after retrieval, vein segments were fixed with buffered (to pH 7.2) 3% glutaraldehyde, at room temperature, under normal pressure, and without papaverine.
Solutions
The chemical characteristics of Plasma-Lyte solution (Baxter) was as follows: each 100 mL contained 526 mg sodium chloride USP, 502 mg sodium gluconate USP, 368 mg sodium acetate trihydrate USP, 37 mg potassium chloride USP, 30 mg magnesium chloride USP (sodium=140 mEq/L, potassium = 5 mEq/L, magnesium = 3 mEq/L, chloride = 98 mEq/L, acetate = 27 mEq/L, gluconate = 23 mEq/L). The pH of the solution was adjusted with sodium hydroxide to 7.4 (6.5 - 8.0) , and the osmolarity was 294 mOsm/L. Papaverine was added in 5-mL (60 mg /mL) quantities to each 500 mL of Plasma-Lyte solution.
The type and composition of the solutions, in addition to the variability of temperature and pressure in our experiments were chosen based upon MMC cardiac surgical practice recommendations and recent surveys of North America Cardiac Surgery Centers, as well as upon recipes suggested in the current literature.
Endothelial Cell Culture
At the conclusion of the previous procedure, vein samples were collected and transported using the same transport media (IMDM). Then, under a laminar flow hood, the pair of veins (OSVH and ESVH) were put in Petri dishes containing 5 mL of endothelial cell culture medium (IMDM with 25 mM HEPES, 3.024 g/L NaHCO3, 100 U/mL penicillin, 100 μ/mL streptomycin, 15% FBS (Fetal Bovine Serum), 30 μ/mL ECGS, 130 m/mL heparin, and 2 mM L-glutamine).
Each vein was flushed with the indicated solution and divided into two pieces using a sterile scalpel (blade No.15). The proximal piece was used for culture, and the distal one for microscopic analysis (light and electron microscopy, both scanning and transmission). The proximal piece of the vein was slit open so that it lay flat. The luminal surface was scraped with a sterile scalpel (No. 11 blade) using light, single strokes, covering each area only once. Cells that built up on the scalpel blade were shaken off into the endothelial culture medium.24 The vein thereafter was rinsed using the culture medium, and the supernatant containing endothelial cells was then preserved in 25-mL culture flasks. These flasks were then incubated at 37°C and 5% CO2 with a humidifier for the next 72 hours in the incubator.
Light Microscopic Evaluation of Endothelial Cells Viability
The endothelial cells in both groups (OSVH and ESVH) were inspected daily with a Labovert Inverted Transmitted Light Microscope (Letiz, Leica Wild, MP S52) and photographs were taken. Everyday 0.2 mL of the supernant was taken and stained by Trifen Blue dye. The cells were examined under light microscopy for morphological characteristics of viability. The critera of the endothelial cells' status was assesssed primarily with the help of a cytologist looking for cell morphology, cytoplasmic discoloration, cytoplasmic organels, cell wall continunity, nuclear shape, nuclear discoloration, intranuclear inclusions, nuclear membrane integrity, and if possible nucleolar abnormalities. A training curve was established for reading the cultured cells, which was supervised, and few of the earliest specimens were not included in the study. We used a haemodiometer to count both viable and dead cells on four successive days, including the day of mechanical harvesting immediately after incubation in the culture medium (day 0). The proportion of viable cells (PVC) was calculated using the following formula:
PVC (%) = No. of viable cells x100 / (No. of viable cells + No. of dead cells).
Institutional Approval
The Institutional Review Board at MMC approved this study.
Statistical Analysis
We used the SAS system (Copyright 1989-1996 by SAS Institute Inc., Cary, NC) to perform the statistical analysis. Data were analyzed with the Wilcoxon Rank-Sum non-parametric test for comparison of medians, and the generalized linear models procedure for comparison of multiple sample means.
RESULTS
Vein Harvesting Technique
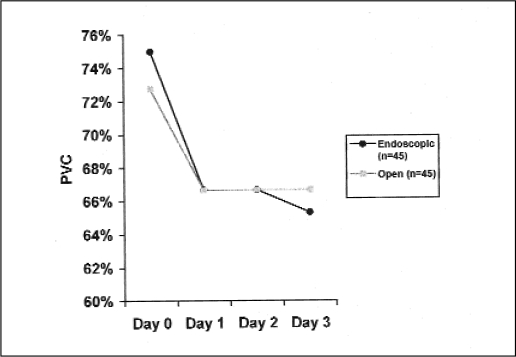
To study whether saphenous vein endothelial cell viability is affected by the technique of vein harvesting, we compared the median PVC from ESVH to that from OSVH, irrespective of incubation temperature, incubation pressure, and the presence of papaverine in the culture medium. Median PVC for the ESVH over days 0 to 4 were 75%, 66.7%, 66.7%, and 65.3%, respectively. The corresponding results for the OSVH were 72.7%, 66.7%, 66.7%, and 66.7%, respectively. The PVC in both techniques showed a slight decline from day 0 to day 1, and then a plateau for the following study days (Figure 2). The medians of PVC for each day were not significantly different between the two techniques (P > 0.005) (Table 2).
Figure 2.
Profile of the median proportion of viable endothelial cells (PVC) between the endoscopic and open methods over the study period.
Table 2.
Comparison of the median proportion of viable endothelial cells between the endoscopic vs. open methods over the study period
| Harvesting technique | Day 0 | Day 1 | Day 2 | Day 3 |
|---|---|---|---|---|
| Endoscopic (n=45) | 75% | 66.67% | 66.67% | 65.3% |
| Open (n=45) | 72.73% | 66.67% | 66.67% | 66.67% |
| P = 0.8341 | P = 0.8616 | P = 0.279 | P = 0.16 |
The Effect of Incubation Temperature
To determine the effect of temperature variation on saphenous vein endothelial cell viability, we compared the means of PVC for each technique, among three different levels of incubation temperature: 4°C, 28°C, and room temperature, irrespective of incubation pressure or the presence of papaverine in the culture medium. The mean PVC under each level of temperature was estimated as the average of individual means for days 0 to 4. In ESVH, no significant difference existed between the mean PVC under room temperature (70.3% ± 3.8%) and that under 4°C (69.5% ± 6.5%) (P > 0.05). However, both results were significantly higher than that under 28°C (56.4% ± 13.3%) (P = 0.0003). In the OSVH, no significant difference existed between the mean PVC under room temperature, 4°C, and 28°C [66.5% ± 3.9%, 71.9% ± 7.2%,63.5% ± 15.7%, respectively (P = 0.07)] (Table 3).
Table 3.
Proportions of endothelial cell viability (mean ± SD): effect of incubating temperature.
| Temperature | ESVH | OSVH |
|---|---|---|
| 4°C | 69.5 ± 6.5% | 71.9 ± 7.2% |
| 28°C | 56.4 ±13.3% | 63.5 ± 15.7% |
| Control (Room Temp) | 70.3 ± 3.8% | 66.5 ± 3.9% |
| P = 0.0003 | P = 0.07 |
The Effect of Distending Pressure
To determine the effect of distending pressure on saphenous vein endothelial cell viability, we compared the means of PVC for each technique, among three different levels of distension pressure: 100 mm Hg, 300 mm Hg, and no pressure, irrespective of incubation temperature or presence of papaverine in the culture medium. The mean PVC under each level of pressure was estimated as the average of individual means for days 0 to 4. In the ESVH, no significant difference existed between the mean PVC under no pressure, 100 mm Hg, and 300 [70.3% ± 3.8%, 63.2% ± 10.6%, and 63.4% ± 13.9%, respectively (P = 0.46)]. Results for the OSVH were similar with the mean PVC under no pressure, 100 mm Hg, and 300 mm Hg being not statistically significant [66.5% ± 3.9%, 68.4% ± 11.4%, and 67.4% ± 14.1%, respectively (P = 0.94)] (Table 4).
Table 4.
Proportions of endothelial cell viability (mean ± SD): effect of distension pressure.
| Pressure | ESVH | OSVH |
|---|---|---|
| 100 mm Hg | 63.2 ± 10.6% | 68.4 ± 11.4% |
| 300 mm Hg | 63.4 ± 13.9% | 67.4 ± 14.1% |
| Control | 70.3 ± 3.8% | 66.5 ± 3.9% |
| P = 0.46 | P = 0.94 |
The Effect of Papaverine
To determine the effect of the addition of papaverine on saphenous vein endothelial cell viability, we compared the means of PVC for each technique, in the presence and absence of papaverine in the culture medium, irrespective of incubation temperature or distention pressure. The mean PVC for each papaverine group was estimated as the average of individual means for days 0 to 4. In the ESVH, no significant difference existed between the mean PVC in the presence of papaverine and that without papaverine [67.3% ± 8.5% and 61.7% ± 13.2%, respectively (P = 0.12)]. However, the results for the OSVH were significantly different with the mean PVC with papaverine (72.5% ± 9.8%) being higher than that without papaverine (64.3% ± 12.4%) (P = 0.019) (Table 5)
Table 5.
Proportions of endothelial cell viability (mean ± SD): effect of papaverine.
| Papaverine | ESVH | OSVH |
|---|---|---|
| Yes | 67.3 ± 8.5% | 61.7 ± 13.2% |
| No | 72.5 ± 9.8% | 64.3 ± 12.4% |
| P = 0.12 | P = 0.019 |
DISCUSSION
Endothelial cells collected by mechanical harvest have been particularly useful for studies of cell viability, when cultured under different physical and chemical conditions.25 Endothelial cell viability as a predictor of cell integrity and future vein patency has had many applications in basic science research.5 Therefore, the importance for continued patency of preserving the endothelial lining of the vein graft used for CABG has been emphasized in many studies over the past two decades.26,27
Although earlier studies did not examine the underlying physiologic and biochemical mechanisms extensively,1 it was understood that steps involved in the preparation and handling of the saphenous vein were critically important and had a major impact on the eventual health and patency of the constructed bypass conduit.27,28 The assumption was that a vein graft with normal morphology at the time of reimplantation had the greatest likelihood of adapting to the arterial flow without subsequent lumen reduction due to thrombus or intimal fibromuscular lesion formation.1 These steps include: 1) techniques of vein harvesting (OSVH or ESVH), 2) vein handling (including distending pressure), 3) vein preservation (temperature, type of solution, additives), 4) use of vitamin K antagonists and platelet inhibitors,29 and 5) techniques of vein reimplantation and anastomosis.29
Many investigators focused on the concept of avoiding direct trauma to vein grafts during dissection or distention. In addition, they stressed the fact that normal vein walls were very sensitive to manipulation and virtually any degree of trauma producing endothelial shedding and vigorous, prolonged contraction of the vein wall.1,30–32 Some lost endothelial cells can be replaced in arteries and in veins used as arterial bypass grafts within weeks,33,34 and loss of endothelium in a vessel may not necessarily be detrimental to patency in the face of adequate flow rates. In those vein grafts in which flow is abnormally slow or borderline due to poor run-off, however, retention of the endothelial lining may be essential for continued graft patency.1
Much of the focus in the literature has been on the importance of vein handling, preservation relevant to the open, no-touch technique of vein harvesting. In 1985, Meldrum-Hanna et al34 described a new technique of saphenous vein harvesting that was a version of the standard well-known open method at that time that used multiple small incisions and a Mayo “stripper” rather than the single “long cut,” thus minimizing major wound complications associated with it. They concluded at the end of their study that vein harvesting by their method was easy and had good results, both morphologically and functionally. In addition, patients had minimal wound complications and superior cosmetic results. Later in 1996, Allen et al35 described a further development with the introduction of the endoscopic technique, focusing on the lower wound complication rates compared with the traditional method and the minimal impact on vein morphology and structure. In contradistinction to older beliefs, ESVH has revolutionized surgical intervention with a uniquely safe vein conduit, both in structure and morphology.36,37
Our study clearly demonstrates that endothelial cell survival for the first 72 hours of both harvesting techniques (ESVH and OSVH) were essentially comparable, regardless of other preservation factors, namely temperature, pressure, and incubating solutions. These findings support results from earlier studies in that despite a perceived compromise in the quality of the operative procedure and postoperative results, ESVH remains a minimally-invasive procedure with levels of endothelial injury comparable to those in the traditional method but with less patient morbidity.9
Major disagreement exists about the ideal environment in which the harvested vein has to be preserved. Extreme cold (4°C) can result in endothelial sloughing and denudation secondary to venospasm.38 Furthermore, a low temperature could derange enzyme-catalyzed reactions that are sensitive to temperature changes, and cellular electrolyte imbalance could result from extreme hypothermia.39 Therefore, it has been suggested that temperatures 10 to 20°C are more favorable for vein preparation. The results in our study showed a significantly better cell viability fraction at 4°C and also at room temperature, compared with that at 28°C in ESVH, whereas viability did not differ significantly for the OSVH. We believe that our findings indicate a real advantage for a lower incubation temperature, and that the inconsistency of results between the two techniques may be a statistical artifact created by steep differences among PVC values.
No differences in PVC existed for any pressure group in our study, regardless of any other factor. This is in agreement with previous studies, which showed that distension pressures of up to 500 mm Hg were not harmful to vein ultrastructure,38 supporting the fact that brief mechanical stress had no significant impact on the vein during preparation.
Our study also investigated the effect of adding papaverine to the preservation solution. Our results indicate a slight advantage for adding papaverine in the OSVH method, but not for the ESVH. Papaverine has been used to prevent graft spasm by incubating the vein in a solution containing papaverine hydrochloride, or by perivenous graft infiltration with it.38 The effect of papaverine is particularly evident when applied intraluminally causing a marked increase in free flow of the artery.40 Papaverine not only minimizes endothelial injury but also allows proper arterialization of the implanted veins, by augmenting the fibrocellular layer in the intima and media, to produce a conduit closely resembling an artery.23 Moreover, Plasma-Lyte containing papaverine has been found to be a better physiological buffering media for saphenous vein conduits, regardless of the method of harvesting and variation in temperature and pressure.40 However, it has also been reported that solutions containing papaverine are toxic to smooth muscle cells, and the vasodilator property might be related to smooth muscle death within the conduit.2
Our study has many limitations that might influence valid interpretation of results. The number of patients and therefore the number of vein samples taken were relatively small. This was further reduced by the necessary subgroupings. The comparisons between different levels of temperature and pressure were unbalanced and might have affected the efficiency of the study. The small counts obtained for viable and dead cells did not allow precise PVC values and resulted in abrupt differences among them. Although endothelial cell viability has been shown to correlate with patency, the long-term effect on graft patency and survival and the contribution of other adverse factors remain to be verified.
CONCLUSION
The introduction of the novel endoscopic method for saphenous vein harvesting for CABG has raised major concerns about possible adverse effects on the integrity of the endothelial lining and graft survival. In addition, important disagreement exists about the optimal preparation method of the retrieved vein. Our findings suggest that endothelial cell viability is not compromised significantly by the endoscopic technique and therefore offers a great advantage to the patient because of its lower postoperative complications. The temperature of the solution at which the vein graft is preserved appears to have a major influence on subsequent endothelial cell viability, regardless of other factors. Low temperatures seem to result in a better cell survival. Whereas distension pressure had no significant effect on viability, the effect of adding the substance papaverine appeared to be inconsistent. We recommend the endoscopic method for harvesting of the saphenous vein for its ease, minimal invasiveness, and lower postoperative morbidity for the patient. We also believe that brief mechanical stress on the vein has no significant adverse effect, provided that the vein is preserved in an appropriate solution under low temperature. Further studies are required to address the effect of papaverine on endothelial cell survival.
Acknowledgment:
This project was supported by a grant from the Maimonides Medical Center Research Foundation.
Contributor Information
Sadir J. Alrawi, Department of Surgery, Maimonides Medical Center, Brooklyn, New York, USA; Departments of Research, Maimonides Medical Center and Lutheran Medical Center Brooklyn, New York, USA.
Ramanathan Raju, Department of Surgery, Maimonides Medical Center, Brooklyn, New York, USA; Departments of Research, Maimonides Medical Center and Lutheran Medical Center Brooklyn, New York, USA.
Anthony J. Acinapura, Division of Cardiothoracic Surgery, Maimonides Medical Center Brooklyn, New York, USA.
Joseph N. Cunningham, Jr, Division of Cardiothoracic Surgery, Maimonides Medical Center Brooklyn, New York, USA.
References:
- 1. Cunningham JN, Spencer FC. Vein contraction and smooth muscle cell extension as causes of endothelial damage during graft preparation. Ann Surg. 1981;194:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gryglewski R, Bunting S, Moncada S, Flower RJ, Vane RJ. Arterial walls are protected against deposition of platelet thrombi by a substance (Prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976;12:685–713 [DOI] [PubMed] [Google Scholar]
- 3. Logerfo FW, Quist WC, Crawshaw HM, Haudenschild C. An improved technique for preservation of endothelial morphology in vein grafts. Surgery. 1981;90:1015–1023 [PubMed] [Google Scholar]
- 4. Todd AS. Endothelium and fibrinolysis. Atheroscleosis. 1972;15:137–140 [DOI] [PubMed] [Google Scholar]
- 5. Rubens FD, Labow RS, Meek E, et al. Papaverine solutions cause loss of viability of endothelial cells. J Cardiovasc Surg. 1998;39:193–199 [PubMed] [Google Scholar]
- 6. Guo-Wei, Rosenfeldt FL, Angus JA. Pharmacological relaxation of the saphenous vein during harvesting for coronary artery bypass grafting. Ann Thorac Surg. 1993;55:1210–1217 [DOI] [PubMed] [Google Scholar]
- 7. Cable DG, Dearani JA, Pfeifer EA, Daly RC, Schaff HV. Minimally invasive saphenous vein harvesting: endothelium integrity and early clinical results. Ann Thorac Surg. 1998;66:139–143 [DOI] [PubMed] [Google Scholar]
- 8. Utly JR, Thomanson ME, Wallace DJ, et al. Postoperative correlates of impaired wound healing after saphenous vein excision. J Thorac Cardiovasc Surg. 1989;98:147–149 [PubMed] [Google Scholar]
- 9. Hanna MW, Ross D, Johnson D, Deal C. An improved technique for long saphenous vein harvesting for coronary revascularization. Ann Thorac Surg. 1986;42:90–92 [DOI] [PubMed] [Google Scholar]
- 10. Kaufmnan JL, Whittemore AD, Couch NP, Mannick JA. The fate of bypass grafts to an isolated popliteal artery segment. Surgery. 1982;92:1027–1031 [PubMed] [Google Scholar]
- 11. Szilagyi DE, Hageman JH, Smith RF, Elliott JP, Brown F, Dietz P. Autogenous vein grafting in femoropopliteal atherosclerosis: the limit of its effectiveness. Surgery. 1979;86:836–851 [PubMed] [Google Scholar]
- 12. DeWeese JA, Rob CG. Autogenous vein grafts ten years later. Surgery. 1977;2:775–784 [PubMed] [Google Scholar]
- 13. Grondin CM, Meere C, Castonguay YR. Blood flow through aorta-to-coronary artery bypass grafts and early postoperative patency. Ann Thorac Surg. 1971;12:574–581 [DOI] [PubMed] [Google Scholar]
- 14. Kahn SP, Lindenauer SM, Dent TL, Kraft RO, Fry WJ. Femorotibial vein bypass. Arch Surg. 1973;107:309–312 [DOI] [PubMed] [Google Scholar]
- 15. Leathr RP, Karmody AM. The saphenous vein for arterial bypass. In: Stanley JC. ed. Biologic and Synthetic Vascular Prostheses New York: Grune & Stratton;1982:351–364 [Google Scholar]
- 16. Metke MP, Lie JT, Fuster V, Josa M, Kaye MP. Reduction of intimal thickening in canine coronary bypass vein grafts with dipyridamole and aspirin. Am J Cardiol. 1979;43:1144–1148 [DOI] [PubMed] [Google Scholar]
- 17. Snyder SO, Gayle RG. Optimal techniques for harvesting and preparation of reversed autogenous vein for use as arterial substitutes. Surgery. 1984;886:96–105 [PubMed] [Google Scholar]
- 18. Wooster DL, Provan JL, Sojka SG, Madras PN. Femoropopliteal bypass: saphenous vein and expanded polytetrafluoroethylene. Can J Surg. 1982;25:666–669 [PubMed] [Google Scholar]
- 19. Abbott WM, Weiland S, Austen WG. Structural changes during preparation of autologous vein grafts. Surgery. 1974;76:1031–1040 [PubMed] [Google Scholar]
- 20. Boncheck LI. Prevention of endothelial damage during preparation of saphenous veins for bypass grafting. J Thorac Cardiovasc Surg. 1980;179:911–915 [PubMed] [Google Scholar]
- 21. Haudenschild CC, Quist WC, Gould KF, LoGerfo FW. Protection of endothelium in vessel segments excised for grafting. Circulation. 1981;64(Suppl. II):101–107 [PubMed] [Google Scholar]
- 22. Gundry SR, Jones M, Ishihara T, Ferrans VJ. Optimal preparation techniques for human saphenous vein grafts. Surgery. 1980;88:785–794 [PubMed] [Google Scholar]
- 23. Sottiurai VS, Sue SL, Batson RC, Frey DJ, Khaw H. Effect of papaverine on smooth muscle cell morphology and vein graft preparation. J Vasc Surg. 1985;834:2–6 [DOI] [PubMed] [Google Scholar]
- 24. Ryan US. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984;56:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ryan US, Mortara M, Whitaker C. Methods for microcarrier culture of bovine pulmonary endothelial cells avoiding the use of enzymes. Tissue Cell. 1980;12:619–635 [DOI] [PubMed] [Google Scholar]
- 26. Gundry ST, Jones M, Ishihara T, Ferrans VJ. Optimal preparation techniques for human saphenous vein grafts. Surgery. 1980;88(6):785–794 [PubMed] [Google Scholar]
- 27. Quist WC, Haundenschild CC, LoGerfo FW. Qualitative microscopy of implanted vein grafts: effects of graft integrity on morphologic fate. J Thorac Cardiovasc. 1992;103:671–677 [PubMed] [Google Scholar]
- 28. Etchberger KJ, Rodewald AJ, Raper BA, Kevorkian MA, Herring MB.Storage condition affect endothelial cell morphology and yields from adult human veins. In Vitro Cell Dev Biol. 1991;27:352–354 [DOI] [PubMed] [Google Scholar]
- 29. Sundt TM., III Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg. 1987;66:172–180 [DOI] [PubMed] [Google Scholar]
- 30. O' Neil JF. The effects on venous endothelium of alterations in blood flow through the vessels in vein walls, and the possible relation to thrombosis. Ann Surg. 1974;126:270–288 [PubMed] [Google Scholar]
- 31. Gottlob R. The preservation of the venous endothelium by “dissecting without touching” and by a traumatic technique of vascular anastomosis. Min Chir. 1977;32:693–700 [PubMed] [Google Scholar]
- 32. Krupski W, Thal ER, Gewertz BL, et al. Endothelial response to venous injury. Arch Surg. 1979;144:1240–1248 [DOI] [PubMed] [Google Scholar]
- 33. Malczak HT, Buck RC. Regeneration of endothelium in rat aorta after local freezing: a scanning electron microscopic study. Am J Pathol. 1977;86:133–148 [PMC free article] [PubMed] [Google Scholar]
- 34. Wyatt AP, Taylor GW. Vein grafts: changes in the endothelium of 15 autogenous free vein grafts used as arterial replacements. Br J Surg. 1966;53:943–947 [DOI] [PubMed] [Google Scholar]
- 35. Allen KB, Griffith GL, Heimansohn DA, et al. Endoscopic versus traditional saphenous vein harvesting: a prospective, randomized trial. Ann Thorac Surg. 1998;66:26–32 [DOI] [PubMed] [Google Scholar]
- 36. David D, Mohammed SF, Woodward SC, Nelson RM. The influence of technique on endothelial preservation in saphenous veins. J Surg Res. 1990;52:219–225 [DOI] [PubMed] [Google Scholar]
- 37. Santoli E, Mattia Boldorini R, Mingoli A, Tosoni A, Santoli C. University of and human saphenous vein graft preservation: preliminary anatomic report. Eur J Cardiothorac Surg. 1993;7:548–552 [DOI] [PubMed] [Google Scholar]
- 37. LoGerfo FW, Haudenschild CC, Quist WC. A clinical technique for prevention of spasm and preservation of endothelium in saphenous vein grafts. Arch Surg. 1984;119:1212–1214 [DOI] [PubMed] [Google Scholar]
- 38. Solberg S, Larsen T, Jorgensen L, Sorlie D. Cold-induced endothelial cell detachment in human saphenous vein grafts. J Cardiovasc Surg. 1987;28:571–575 [PubMed] [Google Scholar]
- 39. Catinella FP, Cuningham JN, Srungaram MD, et al. The factors influencing early patency of coronary artery bypass vein grafts. Thorac Cardiovasc Surg. 1982;83:686–700 [PubMed] [Google Scholar]
- 40. Catinella FP, Cunningham JN, Srungaram MD, et al. The factors influencing early patency of CABC. Thoracic Cardiovascular Surg. 1982;83:686–700 [PubMed] [Google Scholar]