Abstract
It has been shown that a video-laparoscopic approach is the preferred method for treatment of cholecystitis. However, when we consider acute cholecystitis, many questions must be answered. The aim of this study is to compare video-laparoscopic and conventional surgery in the management of acute cholecystitis.
Keywords: Laparoscopy, Acute cholecystitis, Cholecystectomy
INTRODUCTION
Video-laparoscopic cholecystectomy (VLC), which is considered the gold standard for treating gallbladder lithiasis, finds its greatest challenge in acute cholecystitis. VLC has been quickly accepted due to the advantages it provides in the following areas: return to physical activity and work in 5 to 7 days; 1–5 a reduced hospital stay; 1–7 safety due to magnified visualization of intraabdominal structures;2,5,6 low morbidity,1,5,7–15 reduced costs; 4,16 less tissue trauma; a better cosmetic effect; and less pain in the postoperative period, which was observed in almost every published series. For these reasons, many surgeons have adopted this method without carrying out randomized studies. In addition, patients have been stimulated by information about the procedure provided by the media and have begun to demand video-laparoscopic treatment. Conventional surgery, in most cases in Brazil, has been reserved for patients who are not covered by the Health Security program.
The applicability of the laparoscopic method has already been demonstrated in the management of acute disease,17 but the general opinion is that a large number of technical difficulties can be present that increase the need for conversion to open surgery.18–21Even today, few studies are available that compare a laparoscopic approach with the conventional method.22–27 This study is aimed at comparing the laparoscopic approach with conventional surgery for acute cholecystitis.
METHOD
From January 1992 to December 1996, 1182 cholecystectomies were carried out at the General Surgery Service of São Rafael Hospital in the city of Salvador, State of Bahia, Brazil.
The anatomicopathological diagnosis of acute cholecystitis was confirmed in 155 (13.11%) of the patients. Two groups were analyzed prospectively. Group I was formed by the patients who underwent video-laparocholecystectomy (VLC), and Group II comprised patients who underwent open cholecystectomy (OC). The open surgery was performed in patients who were not entitled by their Health Security Plan to undergo the laparoscopic method. We used no other selection criteria.
Open cholecystectomy was carried out with the “standard” technique, through a right subcostal incision, followed by the release of adhesions, dissection of the linking pedicle, sectioning of the cystic artery and cystic duct, and cholecystectomy. If the anatomical dissection of the pedicle was hard to perform, a “fundus first” technique was used.
The VLC operations were performed by the same staff surgeons, using a standard technique (the European method), modified by Dr Enrico Croce, Italy's pioneer of this method.6 The pneumoperitoneum was established through the closed technique, except when abdominal distension was present. The abdominal cavity pressure was maintained below 15 mm Hg. The first trocar (10 mm) was placed via the umbilicus, and the others were placed at the epigastrium (5 mm) to the left of the round ligament, the mesogastrium (10 mm) on a equidistant point between the previously mentioned trocars and to the left of the middle line, and the last trocar was inserted on the right (5 mm) parallel to the umbilicus. A 25 degree laparoscope was used. Dissection of the gallbladder pedicle elements was performed with the aid of blunt dissectors and gauze. This was followed by isolation, clipping, and sectioning of the cystic artery and duct. Most of the time, a standard cholecystectomy was performed, except when anatomic difficulties arose in dissecting the pedicle, and then a “fundus fist” technique was performed.
In all instances, intraoperative cholangiography was performed in patients with anatomical variations, and common bile duct dilatation without preoperative evidence of stones in the common bile duct. When these signs were found before the surgery, endoscopic retrograde cholangiopancreatography (ERCP) or cholangioresonancy (CA) was performed to confirm and remove the stone.
All patients were operated upon within the first 72 hours after admission; they were corrected for fluid and electrolyte imbalance, nausea, vomiting, and pain. A first-generation cephalosporin was used for antibiotic prophylaxis given before the induction of anesthesia. Antibiotic therapy was used in selected cases.
We analyzed the following data: age, sex, previous surgery, surgical risk, signs and symptoms, laboratory evaluation, surgical time, morbidity, mortality, conversion to open surgery, microbiological analysis of aspirated bile, time of hospital stay, and use of drains.
The statistical analysis was performed using the chi-square test, Fisher's exact test, Student's t test, and the Kruskal-Wallis ANOVA test. The results were considered significant with P < 0.05.
RESULTS
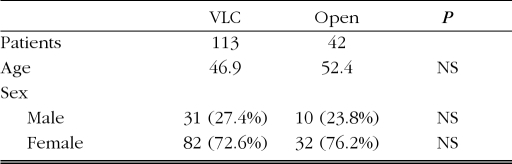
Both groups were predominantly female, with ages ranging from 12 to 90 years (Table 1).
Table 1.
Comparison between VLC vs open cholecystectomy by sex and age.
| VLC | Open | P | |
|---|---|---|---|
| Patients | 113 | 42 | |
| Age | 46.9 | 52.4 | NS |
| Sex | |||
| Male | 31 (27.4%) | 10 (23.8%) | NS |
| Female | 82 (72.6%) | 32 (76.2%) | NS |
VLC=video-laparocholecystectomy.
Patients from both groups underwent other surgeries of the upper abdomen, 1.77% (VLC) and 4.76% (OC). Surgical risk was classified according to the criteria of the American Society of Anesthesiology (Table 2).
Table 2.
Incidence of surgery of the upper abdomen and surgical risk.
| VLC | Open | P | |
|---|---|---|---|
| Previous surgery | 53 (46.9%) | 17 (40.48%) | NS |
| Upper abdominal surgery | 2 (1.77%) | 2 (4.76%) | NS |
| ASA 1 | 56 (49.6%) | 20 (47.6%) | NS |
| ASA 2 | 37 (32.7%) | 14 (33.3%) | NS |
| ASA 3 | 9 (7.96%) | 5 (11.5%) | NS |
| ASA 4 | 1 (0.88%) | 0 (0%) | NS |
| ASA E | 10 (8.85%) | 3 (7.14%) | NS |
ASA=American Society of Anesthesiology; VLC=video-laparocholecystectomy.
Pain in the upper right quadrant, fever, and a palpable gallbladder were the prevalent signs and symptoms, as shown on Table 3.
Table 3.
Frequency of the most common signs and symptoms.
| VLC | Open | P | |
|---|---|---|---|
| Pain URQ | 68 (60.18%) | 24 (57.14%) | NS |
| Fever | 16 (14.16%) | 13 (30.95%) | 0.03 |
| Palpable gallbladder | 13 (11.5%) | 1 (2.38%) | NS |
URQ=upper right quadrant;
VLC=video-laparocholecystectomy.
The required laboratory exams, which included a leuko-gram, amylase, GOT (aspartate aminotransferase, serum), GPT (alanine aminotransferase, serum), bilirubin, and AP (alkaline phosphatase serum), revealed small nonsignifi-cant variations when the two groups were compared (Table 4).
Table 4.
Comparison of laboratory exams between VLC vs open cholecystectomy.
| Laboratory | VLC | Open | P |
|---|---|---|---|
| Leuko > 10 000 | 52 (47.7%) | 25 (61%) | NS |
| Bilirubins > 1 | 25 (22.9%) | 17 (41.5%) | 0.316 |
| GOT | 19 (17.4%) | 12 (29.3%) | 0.49 |
| GPT | 21 (19.3%) | 12 (29.3%) | NS |
| Amylase | 10 (9.2%) | 5 (12.2%) | NS |
GOT=aspartate aminotransferase, serum; GPT=alanine aminotransferase, serum; VLC=video-laparocholecystectomy.
The surgical time for VLC was equal to that of OC and was considered nonsignificant. Cholangiography was carried out in 7.96% of the VLC group compared with 14.28% of Group II (P = 0.247).
A bile culture, was positive in 20.3% in Group I and 16.67% in Group II. The most common pathogens are listed in Table 5. Surgical drainage was used more in Group I, but had no statistical significance (P = 0.074).
Table 5.
Comparison of culture findings between VLC vs cholecystectomy.
VLC= video-laparocholecystectomy.
No intraoperative complications (lesion of the main bile ducts, vascular or intestinal injury, or injury of the hepatic parenchyma) related to surgical technique occurred in either group. The most frequent postoperative complication in Group I was atelectasis (4 cases), followed by respiratory tract infection (RTI), and bilirachia, with 2 cases each. In Group II, the most common complication was RTI, in 4 patients, followed by bilirachia (3 cases), and atelectasis (2 cases). The incidence of complications was greater in Group II (P = 0.006) (Table 6).
Table 6.
Comparison of postoperative complications between VLC vs cholecystectomy.
| Postoperative complications | VLC | Open | P |
|---|---|---|---|
| Atelectasis | 4 (3.53%) | 2 (4.76%) | NS |
| Bilirhachia | 2 (1.76%) | 3 (7.14%) | NS |
| Respiratory | Tract | ||
| Infection | 2 (1.76%) | 4 (9.52%) | 0.04 |
| Subphrenic collection | 1 (0.88%) | 0 | NS |
| Intracavity abscess | 0 | 1 (2.38%) | NS |
| Wall abscess | 0 | 1 (2.38%) | NS |
| Choledocholithiasis | 0 | 1 (2.38%) | NS |
| Wall dehiscence | 0 | 1 (2.38%) | NS |
| Total | 9 (100%) | 13 (100%) | 0.006 |
VLC=video-laparocholecystectomy.
In 14 patients (12.4%) the need to convert to open surgery was due to the presence of adhesions in 5 cases and difficulty in anatomical identification of the pedicle elements (Table 7).
Table 7.
Frequency of determining factors conversions.
| Determining factors | Conversions |
|---|---|
| Adherences | 5 |
| Difficulty for anatomic identification | 5 |
| Cholecystoduodenal fistula | 1 |
| Cystic duct lesion | 1 |
| Choledocholithiasis | 1 |
| Gallbladder necrosis | 1 |
| Total | 14 |
The total mortality was 0.75% (1 case) in Group II due to sepsis and multi-organs system failure (DMOS). The hospital stay was significantly longer in Group II with P = 0.0003782 (Table 8).
Table 8.
Comparison of mean hospital stay and mortality between VLC vs cholecystectomy.
| VLC | Open | P | |
|---|---|---|---|
| Hospital Discharge | 3.67 days | 6.28 days | 0.0003782 |
| Mortality | 0 | 1 (2.38%) | NS |
VLC=video-laparocholecystectomy.
DISCUSSION
The statistical analyses demonstrate that as far as the distribution for sex, age, signs and symptoms, laboratory data, surgical risk, and previous surgeries is concerned, the groups do not have significant differences and can be matched, in spite of the fact that no previous randomiza
In 14 patients (12.4%) the need to convert to open surgery was due to the presence of adhesions in 5 cases and difficulty in anatomical identification of the pedicle elements (Table 7).
The total mortality was 0.75% (1 case) in Group II due to sepsis and multi-organs system failure (DMOS). The hospital stay was significantly longer in Group II with P = 0.0003782 (Table 8).
DISCUSSION
The statistical analyses demonstrate that as far as the distribution for sex, age, signs and symptoms, laboratory data, surgical risk, and previous surgeries is concerned, the groups do not have significant differences and can be matched, in spite of the fact that no previous randomization has been performed. As for age and sex distribution, the data are compatible with reports in the literature.23–26
The signs and symptoms were analyzed with an emphasis on severity indicators. Leukocytosis was present in 55% of patients, a finding compatible with the analysis of 198 cases of acute cholecystitis reported by Grurber28 in 1996. Jaundice occurred in 21.9% of the patients.
A significantly greater fever (>38°C) in the open surgery group was the only factor not matched in the comparative analysis, its total incidence being 18.7% versus 32% found in the Grurber series.
As for the surgical risk, most patients (81.9%) were ASA I and II.28 The ASA 3 and 4 patients were also operated upon with video-laparoscopy. Several reports 29–36 support the use of laparoscopy in the critically ill patient, because the casual deleterious effect of pneumoperitoneum can be promptly corrected. Several authors have reported on the use of laparoscopy for acute cholecystitis, independent of surgical risk,23,25 except in cases of hemodynamic instability.
It is well known that laparotomy leads to the formation of intracavity adhesions. The presence of upper abdominal surgery in 1.7% of the patients did not prevent performance of the laparoscopic method.
The surgical time was similar in both methods, which is compatible with reports in the literature.23,26
The culture of aspirated bile was positive in 19.35% of all patients. Farinon37 in 1993 reported that 29% of patients with acute cholecystitis had positive cultures. E. coli and Klebsiella were the most frequently found pathogens.
Surgical drainage was employed when dissection of the hepatic bed was laborious (bleeding), a common occurrence with acute cholecystitis. Drainage was performed to monitor potential postoperative bleeding and evaluate postoperative bile secretion. Drainage was carried out without incident in both groups (46.7%); reports in literature 23,38 indicate that drainage is performed in 48 to 100% of cases.
Numerous studies 39–43 either support or do not support 44–48 the routine use of intraoperative cholangiography. In the present study, selective cholangiography was carried out in both groups (9.67% of the cases) when intraoperative indications of choledocholithiasis was noted.
No intraoperative complications occurred that might be inherent to the technique. Bickel24 in 1996 reported 1 case of injury of the bile ducts in each group in a total of 182 patients. Cox49 in 1993 reported 1 injury in 98 patients operated on with VLC, and Unger50 in 1994 reported 1 case in 270.
The 3 postoperative complications in both groups could be overlapping, except for the respiratory tract infection, which was more common in Group II. The increased incidence of atelectasis in open surgery of the upper abdomen has already been widely reported. In VLC, diaphragm compression caused by pneumoperitoneum favors, in principle, the appearance of atelectasis.19,49,50 Coelho51 has demonstrated that ventilation dynamics is best after VLC. This fact, together with the occurrence of greater pain in the period following operation in group II, may be factors that facilitate causing respiratory tract infection in this group.
Bilirachia occurred in 2 patients in Group I and in 3 patients in Group II, without any statistical significance, in a total of 3.22% of cases. This same index was reported by Cox49 in 1993. All patients were drained and underwent conservation treatment.
A subphrenic collection took place in 1 VLC patient, without systemic repercussions and was treated in the conservative way. In Group II, 1 patient with intra-cavity abscess was treated though image-guided drainage.
An abdominal wall infection and one total wall dehiscence took place in Group II patients. The low-wall infection incidence with video-surgery has also been largely documented.10,52–55
Hospital discharge was significantly earlier for group I, according to other comparisons. 24–26
Overall mortality was 0.64%. One patient from Group II with severe sepsis evolved to DMOS and died. Mortality in patients with acute cholecystitis and who were operated upon with VLC has varied from 0% to 4%.19,37,49,52–57
CONCLUSION
In spite of its being a nonrandomized study, patients in our study (matched for sex, age, signs and symptoms presenting on admission, laboratory data, and anesthetic risk) who underwent laparoscopic cholecystectomy compared favorably with those who underwent the traditional open technique. The data presented in this study demonstrate that a video-laparoscopic method can be safely performed when acute cholecystitis is present, resulting in low morbidity and mortality rates and shortened hospital stays.
Contributor Information
Paulo C.G. Amaral, Hospital São Rafael, State of Bahia, Brazil.
Euler M. Ázaro Filho, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Manoel P. Galvão-Neto, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Marcos F. Fortes, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Elias L.Q. Souza, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Rogério S.M Alcântara, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
João E.M.T.M. Ettinger, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Adrian B. Regis, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Manoela M. Sousa, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Vinício M. do Carmo, General Surgery Service, Hospital São Rafael, State of Bahia, Brazil.
Pedro A. Santana, Jr., Faculty of Medicine, Federal University of Bahia, Brazil.
Edvaldo Fahel, Faculty of Medicine, Department of Surgery, Federal University of Bahia, Brazil and General Surgery Service, Hospital São Rafael, BA, Brazil.
References:
- 1. The Southern Surgeons Club A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991; 324:1070–1078 [DOI] [PubMed] [Google Scholar]
- 2. Dubois P, Icar P, Berthelot G, Levard H. Coelioscopic, cholecystectomy. Preliminary report of 36 cases. Ann Surg. 1990; 211:260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller IA. Laparoscopic cholecystectomy:passing fancy or legitimate treatment option?. Gastroenterology. 1990; 99:1527–1535 [DOI] [PubMed] [Google Scholar]
- 4. Kum CK3, Epypasch E, Lefering R, Paul A, Neugebauer E, Troidl H. Laparoscopic cholecystectomy for acute cholecystitis: is it really safe? World J Surg. 1996; 20:43–49 [DOI] [PubMed] [Google Scholar]
- 5. Grece, et al. Hospitalization after laparoscopic cholecystectomy. Br J Surg. 1991; 78:160–162 [DOI] [PubMed] [Google Scholar]
- 6. Croce L, Novellino M, Azzola M, Longoni, Crespi G, Faillace G. La videolaparocolecistectomia: trattamento ideale della litiasi biliare? Esperienza su 100 casi consecutivi non selezionati. Chirurgia. 1991; 4:290–294 [Google Scholar]
- 7. Fahel E, Amaral PCG, Ázaro Filho EM, et al. Videolaparocholecystectomy: casuistry of 1000 cases. JSLS. 1998; 2:141–145 [PMC free article] [PubMed] [Google Scholar]
- 8. Bailey RW, Zucker KA, Flowers JL, et al. Laparoscopic cholecystectomy: experience with 375 consecutive cases. Ann Surg. 1991; 214:531–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scott T, Flowers JA, Bailey RW, et al. A review of 12,397 laparoscopic cholecystectomies. Surg Laparosc Endosc. 1992; 2:224–232 [PubMed] [Google Scholar]
- 10. Way LW. Changing therapy for gallstone disease. N Engl J Med. 1990; 18:1273–1274 [DOI] [PubMed] [Google Scholar]
- 11. Baird DR, Wilson JP, Mason EM, et al. An early review of 800 laparoscopic cholecystectomies at a university affiliated community teaching hospital. Am Surg. 1991; 58:206–210 [PubMed] [Google Scholar]
- 12. Cuschieri A, Dubois F, Mouiel J, et al. The European experience with laparoscopy cholecystectomy. Am J Surg. 1991; 161:385–387 [DOI] [PubMed] [Google Scholar]
- 13. Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: results of a national survey of 4,292 hospitals and analysis of 77,604 cases. Am J Surg. 1993; 165:9–14 [DOI] [PubMed] [Google Scholar]
- 14. Larson GM, Vitale GC, Casey J, et al. Multipractice analysis of laparoscopic cholecystectomy in 1,983 patients. Am J Surg. 1992; 163:221–226 [DOI] [PubMed] [Google Scholar]
- 15. Ganey JB, Johnson PA, Prilman PE, et al. Cholecystectomy: clinical experience with a large series. Am J Surg. 1986; 151:352–357 [DOI] [PubMed] [Google Scholar]
- 16. Pedro AR, Guillermo R, Feste JR. Endoscopic laser cholecystectomy. Houston Med. 1989; 5:124–126 [Google Scholar]
- 17. Eldar S, Sabo E, Nash E, Abrahamson J, Matter I. Laparoscopic cholecystectomy for acute cholecystitis: prospective trial. World J Surg. 1997; 21:540–545 [DOI] [PubMed] [Google Scholar]
- 18. Flowers JA, Bailey RW, Zucker KA. Laparoscopic management of acute cholecystitis: The Baltimore experience. Am J Surg. 1991; 161:388–392 [DOI] [PubMed] [Google Scholar]
- 19. Zucker KA, Flowers JL, Bailey RW, Graham SM, Buell J, Imbembo AL. Laparoscopic management of acute cholecystitis. Am J Surg. 1993; 165:508–514 [DOI] [PubMed] [Google Scholar]
- 20. Velasco JM, Vallina VL. A technique for laparoscopic retraction of the acutely inflamed thick-walled gallbladder. Surg Endosc. 1994; 8:809–811 [DOI] [PubMed] [Google Scholar]
- 21. Assaff Y, Matter I, Sabo E, Mogilner JG, Nash E, Abrahamson J, Eldar S. Laparoscopic cholecystectomy for acute cholecystitis and the consequences of gallbladder perforation, bile spillage, and “loss” of stones. Eur J Surg. 1998; 164:425–431 [DOI] [PubMed] [Google Scholar]
- 22. Calhoun PC, Adams LH, Adams MR. Comparision of laparoscopic and minilap cholecystectomy for acute cholecystitis. Surg Endosc. 1994; 8:1301–1304 [DOI] [PubMed] [Google Scholar]
- 23. Kum CK, Goh PMY, Isaac JR, Tekant Y, Ngoi SS. Laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 1994; 81:1651–1654 [DOI] [PubMed] [Google Scholar]
- 24. Bickel A, Rappaport A, Kanievski V, et al. Laparoscopic management of acute cholecystitis. Surg Endosc. 1996; 10:1045–1049 [DOI] [PubMed] [Google Scholar]
- 25. Eldar S, Sabo E, Nash E, Abrahamson J, Matter I. Laparoscopic versus open cholecystectomy in acute cholecystitis. Surg Laparosc Endosc. 1997; 5:407–414 [PubMed] [Google Scholar]
- 26. Kivilouto T, Sirén J, Luukkonen P, Kivilaakso E. Randomised trial of laparoscopic versus open cholecystectomy for acute and gangrenous cholecystitis. Lancet. 1998; 351:321–325 [DOI] [PubMed] [Google Scholar]
- 27. Altaca G, Ozdemir E, Kiliç K, Tokyay R. Laparoscopic cholecystectomy for acute cholecystitis. Surg Laparosc Endosc. 1996; 6:26–28 [PubMed] [Google Scholar]
- 28. Gruber PJ, Silverman RA, Gottesfeld S, Flaster E. Presence of fever and leukocytosis in acute cholecystitis. Ann Emerg Med. 1996; 28:273–277 [DOI] [PubMed] [Google Scholar]
- 29. Popken F, Kuchle R, Heintz A, Junginger T. Laparoscopic cholecystectomy in high risk patients. Chirurg. 1997; 68:801–805 [DOI] [PubMed] [Google Scholar]
- 30. Wittgen CM, Andrus JP, Andrus CH, Kaminski DL. Cholecystectomy. Which procedure is best for the high-risk patient?. Surg Endosc. 1993; 7:377–379 [DOI] [PubMed] [Google Scholar]
- 31. Carroll BJ, Chandra M, Phillips EH, Margulies DR. Laparoscopic cholecystectomy in critically ill cardiac patients. Am J Surg. 1993; 59:783–785 [PubMed] [Google Scholar]
- 32. Voitk AJ. Establishing outpatient cholecystectomy as a hospital routine. Can J Surg. 1997; 40:284–288 [PMC free article] [PubMed] [Google Scholar]
- 33. Portera CA, Compton RP, Walters DN, Browder IW. Benefits of pulmonary artery catheter and transesophageal echocardio-graphic monitoring in laparoscopic cholecystectomy patients with cardiac disease. Am J Surg. 1995; 169:202–206 [DOI] [PubMed] [Google Scholar]
- 34. D'Albuquerque LA, de-Miranda MP, Genzini T, Copstein JL, Oliveira-e-Silva A. Laparoscopic cholecystectomy in cirrhotic patients. Surg Laparosc Endosc. 1995; 5:272–276 [PubMed] [Google Scholar]
- 35. Angrisani L, Lorenzo M, De-Palma G, Sivero L, et al. Laparoscopic cholecystectomy in obese patients compared with nonobese patients. Surg Laparosc Endosc. 1995; 5:197–201 [PubMed] [Google Scholar]
- 36. Safran D, Sgambati S, Orlando R. Laparoscopy in high-risk cardiac patients. SGO 1993; 176:548–54 [PubMed] [Google Scholar]
- 37. Farinon AM, Grande M, Torquati A, D'Antini P. Multivariate analysis for predicting the presence of bacteria in bile in patients with acute cholecystitis. Eur J Surg. 1993; 159:531–534 [PubMed] [Google Scholar]
- 38. O'Rourke NA, Fielding GA. Laparoscopic cholecystectomy in acute cholecystitis. Aust N Z J Surg. 1992; 62:944–946 [DOI] [PubMed] [Google Scholar]
- 39. Shively EH, Wieman TJ, Adams AL, et al. Operative cholangiography. Am J Surg. 1990; 159:380–384 [DOI] [PubMed] [Google Scholar]
- 40. Joyce WP, Keane R, Burke GJ, et al. Identification of bile duct stones in patients undergoing laparoscopic cholecystectomy. Br J Surg. 1991; 78:1174–1176 [DOI] [PubMed] [Google Scholar]
- 41. Pace BW, Cosgrove J, Breuer B, Margolis IB. Intraoperative cholangiography revisited. Arch Surg. 1992; 127:448–450 [DOI] [PubMed] [Google Scholar]
- 42. Phillips EH. Routine versus selective intraoperative cholangiography. Am J Surg. 1993; 165:505–507 [DOI] [PubMed] [Google Scholar]
- 43. Soper NJ, Brunt LM. The case for routine operative cholangiography during laparoscopic cholecystectomy. Surg Clin North Am. 1994; 74:953–959 [PubMed] [Google Scholar]
- 44. Millat B, Atger J, Deleuze A, et al. Laparoscopic treatment for choledocholithiasis: a prospective evaluation in 247 consecutive unselected patients. Hepatogastroenterology. 1997; 44:28–34 [PubMed] [Google Scholar]
- 45. Nies C, Krack W, Lorenz W, et al. Histamine release in conventional versus minimally invasive surgery: results of a randomised trial in acute cholecystitis. Inflamm Res. 1997; 46:S83–S84 [PubMed] [Google Scholar]
- 46. Pasquale MD, Nauta RJ. Selective vs rotine use of intraoperative cholangiography. Arch Surg. 1989; 124:1041–1042 [DOI] [PubMed] [Google Scholar]
- 47. Gerber A. A requiem for the rotine operative cholangiogram (editorial). SGO. 1986; 163:363–364 [PubMed] [Google Scholar]
- 48. Low SC, Ng FC, Yap YL, Chng HC. Operative cholangiography. Singapore Med J. 1992; 33:252–254 [PubMed] [Google Scholar]
- 49. Cox MR, Wilson TG, Luck AJ, Jeans PL, Padbury RTA, Toouli J. Laparoscopic cholecystectomy for acute inflammation of the gallbladder. Ann Surg. 1993; 5:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Unger SW, Edelman DS, Scott JS, et al. Laparoscopic treatment of acute cholecystitis. Surg Laparosc Endosc. 1994; 1:14–16 [PubMed] [Google Scholar]
- 51. Coelho JC, de-Araujo RP, Marchesini JB, et al. Pulmonary function after cholecystectomy performed through Kocher's incision, a mini-incision, and laparoscopy. World J Surg. 1993; 7:544–546 [DOI] [PubMed] [Google Scholar]
- 52. Wilson RG, Macintyre IM, Nixon SJ, Saunders JH, Varma JS, King PM. Laparoscopic cholecystectomy as a safe and effective treatment for severe acute cholecystitis. BMJ. 1992; 305:394–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Croce E, Azzola M, Golia M, Palazzini G. La colecistectomia laparoscopica nelle colecistiti acute. Laparoscopic cholecystectomy in acute cholecystitis. G Chir. 1992; 13:153–155 [PubMed] [Google Scholar]
- 54. Phillips EH, Carroll BJ, Bello JM, Fallas MJ, Daykhovsky L. Laparoscopic cholecystectomy in acute cholecystitis. Am Surg. 1992; 58:273–276 [PubMed] [Google Scholar]
- 55. Rattner DW, Ferguson C, Warshaw AL. Factors associated with successful laparoscopic cholecystectomy for acute cholecystitis. Ann Surg. 1993; 217:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watteville JC, Gayral F, Testas P. Evaluation de la coelioscopie dans le traitement en urgence de la cholécystite aigue. J Chir. 1992; 129:490–491 [PubMed] [Google Scholar]
- 57. Wiesen SM, Unger SW, Barkin SJ, et al. Laparoscopic cholecystectomy: the procedure of choice for acute cholecystitis. Am J Gastroenterol. 1993; 88:334–337 [PubMed] [Google Scholar]