Abstract
Background:
Achalasia is a relatively rare disorder with a variety of treatment options. Although laparoscopic Heller myotomy has become the surgical treatment of choice, little data exist on the overall quality of life of patients undergoing this technique versus standard open approaches.
Methods:
We prospectively evaluated all patients surgically treated for achalasia by a single surgeon. Laparoscopic Heller myotomy consisted of a long (≥ 6 cm) esophageal cardiomyotomy extending at least 2 cm onto the gastric cardia, with a concomitant Dor fundoplication. Patients were evaluated preoperatively and postoperatively for symptoms and quality of life using the SF-36, a standardized, generic quality of life instrument.
Results:
A total of 23 patients were surgically treated: 15 patients had a planned laparoscopic procedure, with 3 conversions; 8 had planned open procedures. Dysphagia resolved in 20 of 21 patients, with 1 patient in the laparoscopic group requiring reoperation due to an inadequate gastric myotomy. Compared with preoperative scores, a statistically significant improvement occurred in the general health domain of the SF-36 (70 to 82, P = 0.04). Compared with that in patients undergoing open surgery, the laparoscopic group had better scores in the domains of physical functioning and bodily pain.
Conclusions:
Laparoscopic Heller myotomy has comparable success to open Heller myotomy, and causes less early detriment to quality of life. This should be the primary treatment in all fit surgical patients with achalasia.
Keywords: Laparoscopy, Achalasia, Heller myotomy
INTRODUCTION
Achalasia is an uncommon, benign disorder of the esophagus with an annual incidence of 1 per 100,000 people.1 It is characterized by a hypertensive, nonrelaxing lower esophageal sphincter (LES) associated with a hypo- or aperistaltic esophageal body. The etiology of this condition is unclear, but reason exists to believe that it may be secondary to abnormal innervation of the myenteric plexus of the esophagus.2 A recent study of 42 resected esophageal specimens from patients with achalasia revealed fewer ganglion cells, and ganglion cells, when present, surrounded by mononuclear cells, suggesting a cause of ganglion degeneration.3
Hypotheses for acquired causes include viral infections like herpes zoster4 or measles,5 or an autoimmune pathogenesis based on a description of antimyenteric neuron antibodies.6
Regardless of the cause of achalasia, its presentation is quite characteristic. Patients usually present with dysphagia, chest pain, regurgitation, weight loss, and occasionally heartburn. In fact, achalasia can be misdiagnosed as gastroesophageal reflux disease. The dysphagia is progressive and is associated with worsening esophageal dilation. The diagnosis is confirmed with an esophagogram, esophagoscopy, and esophageal manometry.
Management of achalasia is directed at alleviation of symptoms, because currently no therapy exists for the underlying neuropathology. Symptomatic improvement with medical management is poor.2 The mainstays of treatment are pneumatic dilation, 2, 6 endoscopic botulinum toxin injections, 2, 7 and surgical myotomy of the gastroesophageal junction–the so-called Heller myotomy. 2, 8 Surgical intervention was traditionally performed through a left thoracotomy. However, with the popularization of laparoscopy, especially in relation to antireflux procedures, this approach has gained popularity. Nevertheless, although a plethora of studies report symptomatic outcomes of laparoscopic or thoracoscopic Heller myotomies, 2, 8 none has used a validated quality of life instrument to assessment outcomes. We report here our experience with both open and laparoscopic Heller myotomy, with prospectively gathered quality of life outcomes.
PATIENTS AND METHODS
All patients surgically treated for achalasia by a single surgeon (VV) at Henry Ford Hospital over the period of September 1996 to December 2000 were eligible for the study. Achalasia was documented preoperatively in all patients by symptoms, upper gastrointestinal series, upper endoscopy, and esophageal manometry. All treatments prior to surgical intervention were recorded.
Preoperatively, patients completed the SF-36, a generic quality of life instrument. 8–11 This instrument has been used in a variety of surgical outcome studies 12–14 and was therefore considered appropriate for this study. The SF-36 measures 8 “domains” of quality of life: physical functioning (PF), role-physical (RP), role-emotional (RE), bodily pain (BP), vitality (VT), social functioning (SF), mental health (MH), and general health (GH). All scores are standardized so that the worst possible score is 0, and the best possible score is 100 is each domain. The SF-36 does not measure a “grand total” quality of life score. At 6 weeks postoperation, patients once again completed this instrument.
The selection criteria for offering patients a laparoscopic versus open Heller myotomy were as follows: patients with an associated epiphrenic diverticulum, patients with a previously failed Heller myotomy, patients with associated intraabdominal pathology requiring surgical intervention not amenable to a laparoscopic approach, or patients in whom a laparoscopic approach did not seem feasible. All other patients, including those who underwent prior pneumatic dilation or botulism toxin injections, were offered a laparoscopic Heller myotomy.
Laparoscopic Heller myotomy was performed through 5 ports placed in a “diamond” fashion as described by Hunter et al.15 The phrenicoesophageal ligament was divided to expose the gastroesophageal junction; however, dissection in this area was kept to a minimum. The esophageal fat pad was excised. The myotomy was begun on the esophagus with a hook cautery until the submucosal plane was identified. Care was taken to divide both the longitudinal and circular fibers of the esophageal musculature. The myotomy was extended at least 6 cm along the esophagus and 2 cm onto the gastric cardia. The length of the myotomy was controled by intraoperative esophagogastroscopy to ensure completeness of the myotomy. The operation was completed with an anterior 180 degree Dor fundoplication.
The open Heller myotomy was performed through an upper, midline incision. The details of the operation after this were the same as those for the laparoscopic approach, except for 3 patients who had transthoracic Heller myotomies. These 3 patients did not receive a concomitant antireflux procedure. All patients were studied with water-soluble or barium esophagogram, or both of these, postoperatively to rule out occult leaks and document resolution of the “bird's beak.”
Statistical analysis was done with the True Epistat16 statistical computer program. As the data did not follow a Gaussian distribution, they were analyzed nonparametrically using the Mann-Whitney U test. A P-value of 0.05 was considered significant.
RESULTS
A total of 23 patients were surgically treated for achalasia during this period. Eight patients had previous treatment with pneumatic dilation, and 3 were treated with endoscopic injection of botulinum toxin. Fifteen patients had laparoscopic Heller myotomy with Dor fundoplication, 3 of which required conversion to an open procedure. Eight patients underwent a planned open procedure: 2 were re-do myotomies, 3 patients due to associated epiphrenic diverticula, 2 due to obesity, and 1 patient with a concomitant abdominal mass. Three esophageal perforations were recognized intraoperatively, and 1 was repaired laparoscopically. Two of these 3 patients had prior botulinum toxin injections. Two conversions were because of perforations that could not be repaired laparoscopically, and the other was due to an inability to complete an adequate myotomy laparoscopically.
Median length of stay was 2 days in the laparoscopic group, compared with 6 days in the open group (P < 0.05). All patients reported dramatic improvement in their symptoms in the immediate postoperative period. Six weeks later, all but 1 patient in the laparoscopic group still had excellent outcomes. The 1 patient had persistent dysphagia and an esophageal stenosis by a contrast esophagogram consistent with achalasia, which was refractory to repeated pneumatic dilation. At reexploration, it was discovered that the myotomy onto the gastric cardia was incomplete. After extending the myotomy and adding a Dor fundoplication, this patient had complete symptomatic relief.
Two patients in the transthoracic group eventually developed reflux requiring re-operation and a Dor fundoplication. Another patient in this group developed a stricture that responded to pneumatic balloon dilation.
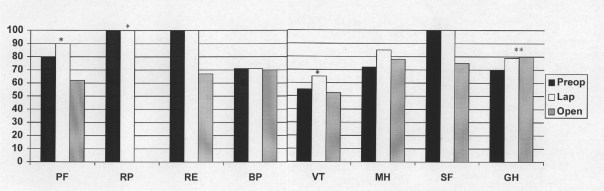
In addition to patients' verbal assessments of their symptoms, each patient at 6 weeks follow-up completed the SF-36. Figure 1 presents the median scores. Compared with the open group, the laparoscopic group had better scores in the domains of physical functioning (PF), role-physical (RP), and vitality (VT) [P ≤ 0.05 for all comparisons]. Compared with preoperative scores, both the laparoscopic and open groups showed improvement in the general health (GH) domain [P = 0.04].
Figure 1.
Median preoperative and postoperative SF-36 scores for patients undergoing laparoscopic and open Heller myotomy with Dor fundoplication. PF = Physical functioning; RP = Role physical; RE = Role-emotional; BP = bodily pain; VT = vitality; MH = mental health; SF = social functioning; GH = general health. * denotes statistically significant differences between the laparoscopic and open groups (P ≤ 0.05). ** denotes statistically significant difference between preoperative and postoperative scores (P < 0.05).
DISCUSSION
Like laparoscopic antireflux procedures, laparoscopic Heller myotomy has gained popularity as the surgical procedure of choice for achalasia. In fact, many gastroenterologists refer fit patients for surgery without previous attempts at pneumatic dilation or botulinum toxin injection.2 Some have been influenced by the only randomized trial comparing open Heller myotomy with pneumatic dilation, which strongly favored surgical therapy.17 In addition, the perception exists that the laparoscopic approach is “less invasive” than either a laparotomy or thoracotomy for completion of the myotomy. Nevertheless, quantitative data using well-validated quality of life instruments has been lacking to support this assertion. We have shown here that the laparoscopic approach offers similar symptomatic outcomes to that of the open Heller myotomy, but with superior quality of life outcomes in 3 of 8 measured domains. Although this is not a randomized trial and is potentially open to bias, these results are consistent with previously published data comparing quality of life outcomes of open and laparoscopic antireflux procedures.18
Nevertheless, some unsettled questions still exist with respect to laparoscopic Heller myotomy. Firstly, can laparoscopic Heller myotomy be done after pneumatic dilation? We performed 7 myotomies after pneumatic dilation without difficulty. Cosentini et al 19 have also shown that the laparoscopic approach is feasible and effective after pneumatic dilation. Therefore, a previous pneumatic dilation should not deter a surgeon from choosing the laparoscopic approach.
Secondly, can laparoscopic Heller myotomy be done after botulinum toxin injection? Botulinum toxin injection results in fibrosis and obliteration of the submucosal plane between the esophageal mucosa and musculature.20 This results in a much more difficult dissection and a higher incidence of perforation, as demonstrated by the fact that 2 of our 3 intraoperative perforations occurred in patients receiving these injections. Therefore, as recommended by some gastroenterologists, 2, 21 botulinum toxin should be reserved for elderly patients who are not surgical candidates. If a patient has already been treated with this method, the surgeon can anticipate a difficult dissection with a high likelihood of mucosal perforation.
Thirdly, is laparoscopic Heller myotomy effective for patients with dilated, aperistaltic esophagi? Some have suggested that marked dilated esophagi associated with “sigmoid” changes as demonstrated by a contrast esophagogram is an indication for esophagectomy with gastric or colon interposition. 22, 23 Patti et al 24 reported on a group of such patients who have done well after laparoscopic Heller myotomy. Therefore, it would seem reasonable that these patients can be offered a laparoscopic myotomy first; then if this fails, an esophagectomy can be the definitive procedure.
Lastly, should a fundoplication be added to a Heller myotomy? Traditionally, a partial fundoplication has been added to the open, transabdominal Heller myotomy. 8, 25 However, this doctrine has been challenged by some surgeons performing the operation laparoscopically, claiming that this approach causes minimal disruption at the esophageal hiatus and therefore is less prone to reflux. 26, 27 Shiino et al8 in a careful review of the literature, nevertheless, conclude that patients undergoing open or laparoscopic Heller myotomy require a fundoplication, whereas, those treated thoracoscopically may not. It has been our experience that patients who do not undergo a concomitant fundoplication do suffer from reflux symptoms; therefore, it is our policy to perform a fundoplication on all patients.
In conclusion, laparoscopic Heller myotomy produces similar symptomatic results as those of the open approach with better quality of life outcomes. Patients who are fit for surgery should be offered this as the primary and definitive treatment for achalasia. A partial, anterior fundoplication (Dor) should be strongly considered, if not routine, for all patients undergoing a Heller myotomy.
References:
- 1. Little AG. Motility disorders of the esophagus. In: Bell RH, Rikkers LF, Mulholland MW, eds. Digestive Tract Surgery: A Text and Atlas. Philadelphia: Lippincott-Raven Publishers; 1996:27–42 [Google Scholar]
- 2. Spiess AE, Kahrilas PJ. Treating achalasia: from whalebone to laparoscopic. JAMA. 1998; 280: 638–642 [DOI] [PubMed] [Google Scholar]
- 3. Goldblum JR, Whyte RI, Orringer MB, Appelman HD. Achalasia, a morphologic study of 42 resected specimens. Am J Surg Path. 1994; 18: 327–337 [PubMed] [Google Scholar]
- 4. Robertson CS, Martin BAB, Atkinson M. Varicella-zoster virus DNA in the oesphageal myenteric plexus in achalasia. Gut. 1993; 34: 299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones DB, Mayberry JF, Rhoades J, Munro J. Preliminary report of an association between measles virus and achalasia. J Clin Path. 1983; 36: 655–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koshy SS, Nostrant TT. Pathophysiology and endoscopic balloon treatment of esophageal motility disorders. Surg Clin North Am. 1997; 77: 971–992 [DOI] [PubMed] [Google Scholar]
- 7. Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996; 110: 1410–1415 [DOI] [PubMed] [Google Scholar]
- 8. Shiino Y, Filipi CJ, Awad ZT, Tomonaga T, Marsh RE. Surgery for achalasia: 1998. J Gastrointest Surg. 1999; 3: 447–455 [DOI] [PubMed] [Google Scholar]
- 9. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): conceptual framework and item selection. Med Care. 1992; 30: 473–483 [PubMed] [Google Scholar]
- 10. McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993; 31: 247–263 [DOI] [PubMed] [Google Scholar]
- 11. McHorney CA, Ware JE, Lu JFR, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994; 32: 40–66 [DOI] [PubMed] [Google Scholar]
- 12. Temple PC, Travis B, Sachs L, Strasser S, Choban P, Flancbaum L. Functioning and well-being of patients before and after elective surgical procedures. J Am Coll Surg. 1995; 181: 17–25 [PubMed] [Google Scholar]
- 13. Velanovich V. Using quality-of-life instruments to assess surgical outcomes. Surgery. 1999; 126: 1–4 [DOI] [PubMed] [Google Scholar]
- 14. Velanovich V. Experience with a generic quality of life instrument in a general surgical practice. Int J Surg Invest. 2000; 1: 447–452 [PubMed] [Google Scholar]
- 15. Hunter JG, Trus TL, Branum GD, Waring JP. Laparoscopic Heller myotomy and fundoplication for achalasia. Ann Surg. 1997; 225: 655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gustafson TL. True Epistat, 4th Ed Richardson, TX: Epistat Services, 1991 [Google Scholar]
- 17. Csendes A, Braghetto I, Henriquez A, Cortes C. Late results of a prospective randomized study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989; 30: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velanovich V. Comparison of symptomatic and quality of life outcomes of laparoscopic versus open antireflux surgery. Surgery. 1999; 126: 782–789 [PubMed] [Google Scholar]
- 19. Cosentini E, Berlakovich G, Zacherl J, et al. Achalasia: results of myotomy and antireflux operation after failed dilatations. Arch Surg. 1997; 132: 143–147 [DOI] [PubMed] [Google Scholar]
- 20. Eaker EY, Gordon JM, Vogel SB. Untoward effects of esophageal botulinum toxin injection in the treatment of achalasia. Dig Dis Sci. 1998; 42: 724–727 [DOI] [PubMed] [Google Scholar]
- 21. Castell DO, Katzka DA. Botulinum toxin for achalasia: to be or not to be? Gastroenterology. 1996; 110: 1650–1652 [DOI] [PubMed] [Google Scholar]
- 22. Ellis FH., Jr Esophagectomy for achalasia: who, when, and how much? Ann Thorac Surg. 1989; 47: 334–335 [DOI] [PubMed] [Google Scholar]
- 23. Peters JH, Kauer WKH, Crookes PF, Ireland AP, Bremner CG, DeMeester TR. Esophageal resection with colon interposition for end-stage achalasia. Arch Surg. 1995; 130: 632–637 [DOI] [PubMed] [Google Scholar]
- 24. Patti MG, Feo CV, Diener U, et al. Laparoscopic Heller myotomy relieves dysphagia in achalasia when the esophagus is dilated. Surg Endosc. 1999; 13: 843–847 [DOI] [PubMed] [Google Scholar]
- 25. Bonavina L, Nosadini A, Bardini R, Baessato M, Peracchia A. Primary treatment for achalasia: long-term results of myotomy and Dor fundoplication. Arch Surg. 1992; 127: 222–226 [DOI] [PubMed] [Google Scholar]
- 26. Shimi S, Nathanson LK, Cuschieri A. Laparoscopic cardiomyotomy for achalasia. J R Coll Surg Edinb. 1991; 36: 152–154 [PubMed] [Google Scholar]
- 27. Wang PC, Sharp KW, Holzman MD, Clements RH, Holcomb GW, Richards WO. The outcome of laparoscopic Heller myotomy with antireflux procedure in patients with achalasia. Am Surg. 1998; 64: 515–521 [PubMed] [Google Scholar]