Abstract
Objective:
To assess the indications and limits of laparoscopic myomectomies (LM).
Methods:
We conducted a retrospective analysis of 89 consecutive cases of LM. Our LM procedures were as follows: Diluted vasopressin was injected into the myoma capsule, and a transverse incision was made by fine monopolar electrode. Traction was applied to the myoma with a myoma screw. The uterine wall was sutured with a curved needle. Fibrin glue spray was applied to prevent adhesion formation. Enucleated myomas were removed via trocar by using an electric morcellator.
Results:
We enucleated 195 nodules with diameters > 2 cm; the mean size of the dominant myomas was 5.3 cm. The mean number of myomas removed from each patient was 2. The uterine wall was sutured in all cases with a mean of 9 sutures. The mean blood loss was 102 mL, and the mean operating time was 111 minutes. No patients were converted to laparotomy. The average hospital stay was 2.4 days. When the myomas were larger than 10 cm, the blood loss and operating time were increased. However, the number of myomas did not correlate with blood loss.
Conclusion:
LM appears to offer a number of advantages if the myoma is not larger than 10 cm.
Keywords: Laparoscopic myomectomy, Surgical technique, Indication, Limitation
INTRODUCTION
Recently, the primary approach in young patients with large or symptomatic myomas who desire a future pregnancy has been laparotomy. However, in select cases, laparoscopic myomectomy (LM) has been reported to be an effective technique that is associated with the development of operative laparoscopic equipment and surgical techniques.1,2 The technical difficulties of LM are controlling bleeding during the enucleation phase of myomectomy, reapproximation of the uterine wall with suturing techniques, and extraction of solid myomas from the abdominal cavity. The benefit of LM and the indication and limitations of laparoscopic myomectomy as a routine treatment are not obvious. The purpose of this study was to assess the limitations, feasibility, risks, and benefits of LM.
MATERIALS AND METHODS
From January 1998 to December 1999, 112 premenopausal women underwent LM. In 13 of these women, the myomas were < 3 cm; and in 10, the myomas were pedunculated. These 23 women were excluded from this study. Only those patients with submucosal, intramural, and subserosal myomas larger than 3 cm (n=89) were included in this study.
For these 89 patients, the indications for the myomectomy, which were sometimes all present in the same patient, were as follows: infertility (n=40; 36.4%); chronic pelvic pain (n=10; 9.1%); severe menometrorrhagia (n=11; 10%); dysmenorrhea (n=24; 21.8%); compression syndrome (n=16; 14.5%); or adnexal mass (n=9; 8.2%). The average age of the patients was 35.2 ± 5.6 years (range 19 to 52). In all patients, the presence of a myoma was confirmed clinically by pelvic and transvaginal ultrasonographic examination. The size of the myoma was measured by ultrasound with myoma size ranging from 3 cm to 16 cm. In 80 patients, the location of the myomas was evaluated by MRI. Gonadotropin releasing hormone agonists (GnRHa) were prescribed in 61 cases (68.5%). Treatment consisted of intramuscular deposit of leuprolide acetate (Leuprin 1.88; Takeda, Osaka, Japan) or buserelin acetate (Suprecur MP; Aventis Pharma, Tokyo, Japan), administered once a month for 3 to 6 months. This preoperative treatment was used for large myomas (>5 cm), anemia, or complicated endometriosis.
Laparoscopic Technique
Anesthesia and Preoperative Settings:
All procedures were performed with the patient under general anesthesia. The patients were prepared for lithotomy, and the Uterine Manipulator™ (Ethicon, Tokyo, Japan) was placed into the uterine cavity for mobilization of the uterus.
Positioning of the Trocar (Figure 1):
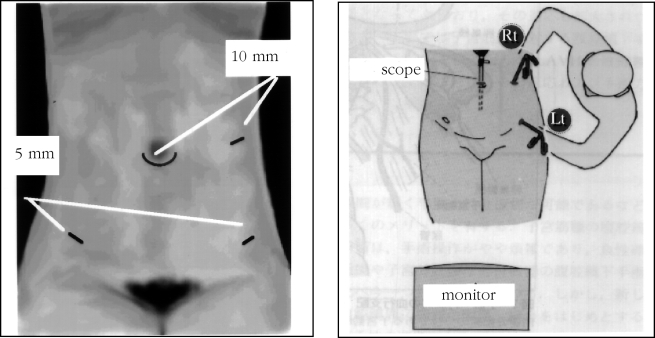
Figure 1.
The sites of trocar insertion and position of the operator. The 10-mm trocar in the left upper quadrant is controlled by the right hand; the left lower 5-mm trocar is manipulated by the left hand.
The laparoscopic procedures were performed with the closed method. After pneumoperitoneum was established, a 10-mm scope was inserted periumbilically. Three additional puncture sites were made as follows: two 5-mm sites 2 cm above the anterior superior iliac supine; and a 10-mm site 3 cm above the umbilicus on the left anterior axial line, an ideal laparoscopic position providing a large operative field. The operator stood on the patient's left side, controlling the 10-mm trocar with his right hand, and manipulating the left lower 5-mm trocar with his left hand.
Surgical Procedure
All procedures were performed by the same surgeon (HT) using a similar technique. Vasopressin was injected between the myometrium and myoma capsule at a concentration of 20 IU/100 mL diluted saline solution prior to excision to reduce the size of the blood vessels and bleeding. The second assistant controlled the uterine manipulator, using an anteverted position for a posterior myoma, or retroverted position for an anterior myoma. The myoma was maintained in a central area of the operation field (Figure 2). A horizontal incision was usually made with a monopolar needle. A grasping forceps, monopolar needle, and myoma screw inserted into the left upper trocar port were used to remove the myoma from its capsule. Traction on the myoma, combined with use of the uterine manipulator and myoma screw, facilitated dissection. After the myoma had been enucleated, hemostasis of the uterine bed was completed with a monopolar needle.
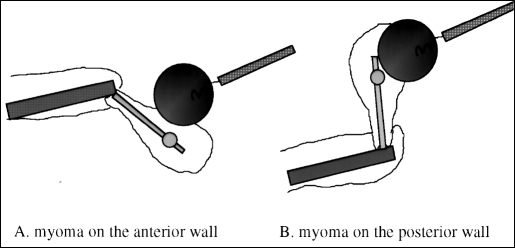
Figure 2.
The myoma is kept in place centrally in the operating field by using the Uterine manipulator and myoma screw to control it. The uterus is manipulated in the retroverted position for an anterior myoma (A) and in the anteverted position for a posterior myoma (B).
The uterine wall was sutured from 1 to 3 layers, according to the depth of the hysterotomy. A 5-mm needle holder with 2/0 Vicryl (Ethicon, Tokyo, Japan) was used to reconstruct the perimetrium with a separate stitch. Sutures are normally placed every 5 mm along the hysterotomy. The edges of the serosa were reapproximated with a baseball suture by Endostitch™ (Auto Suture Tokyo, Japan). Almost all myomas were extracted from the abdominal cavity with the electric morcellator (Karl Storz, Tokyo, Japan). Fibrin glue spray (Aventis Pharma, Tokyo, Japan) was used to prevent postoperative adhesion formation. Forty-one patients underwent one or more additional surgical procedures at the time of myomectomy including cystectomy (n=31); lysis of complete cul-de-sac obliteration (n=6); salpingostomy (n=5); and salpingo-oophorectomy (n=3).
Data were statistically evaluated with the Statistics Package for Social Sciences (SPSS) 10.0 program for Windows (SPSS Inc., Chicago, USAIL). The operating times and estimated blood loss were compared with analysis of variance (ANOVA) based on myoma location. The correlation between operation time, estimated blood loss, dominant myoma size, and the number of removed myomas were determined with the Spearman rank method. Statistical significance was assessed based on P<0.05 and a 2-tailed test.
RESULTS
Laparoscopy was used to enucleate 191 myomas >2 cm in diameter. Of these, 114 myomas were intramural, 75 subserosal, and 7 submucosal. The mean number of removed myomas per patient was 2.2± 1.7 (range, 1 to 9). The number of myomas per patient was 2 (14 cases, 15.7%), 3 (10 cases, 11.2%), and 4 or more (17 cases, 19.2%). The mean size of the dominant myomas was 5.4± 2.3 cm (range, 3.0 to 16). The myoma size was <5 cm in 43 cases (48.3%), between 5 and 10 cm in 42 cases (47.2%), and >10 cm in 4 cases (4.5%). The largest myoma enucleated was intramural in 59 cases (66.3%), subserosal in 23 cases (25.8%), and submucosal in 7 cases (7.9%). The dominant myoma removed was located in the posterior wall in 42 cases (47.2%), in the anterior wall in 31 cases (34.8%), at the fundus in 14 cases (15.7 %), and in the broad ligament in 2 cases (2.3%). The uterine wall was sutured in all cases with a mean suturing number (except when using the Endostitch™ suture) of 9.7 (range, 1 to 30). A double layer suture was necessary in 37 cases (41.6%), and more than 3 layers were necessary in 22 cases (24.7%). An endometrial perforation occurred during enucleation in 6 of 7 patients with submucosal myomas. The endometrial layer was sutured with 3/0 Vicryl via laparoscopy.
The mean operative time was 111 ± 40 minutes (range, 50 to 270). Morcellation time for the myomas was 14 ± 20 minutes (range, 5 to 125). The mean weight of enucleated myomas was 87.2 ± 136 grams (range, 10 to 1040). None of the patients required a laparotomy. The estimated blood loss in 89 patients was between 10 and 2500 mL, with a mean of 102 mL. A blood transfusion was required in only 1 patient.
By far, the longest operative time of 270 minutes and the greatest blood loss of 2500 mL occurred in a patient who had a huge intramural myoma 16 cm in diameter, located anteriorly. The weight of the enucleated myoma was 1040 grams. Bleeding from the uterine wall could not be controlled during suturing despite vasopressin injection, and the extraction time was greater than 120 minutes despite the use of an electric morcellator.
Surgical complications were limited to two cases of postoperative fever greater than 38°C after the first 24 hours, and these cases were treated with antibiotics. In both cases, perioperative difficulties had occurred, and a 10-cm and a 16-cm myoma, respectively, were enucleated by LM.
The duration of postoperative hospitalization ranged from 2 to 7 days, with a mean of 2.4 days.
During follow-up 6 months after surgery, 10 pregnancies occurred out of the 34 infertile patients. Four ended with a vaginal delivery at term, and one with a submucosal myoma was delivered by Cesarean. Two pregnancies resulted in first trimester miscarriages. Spontaneous uterine rupture was not observed in any of the pregnancies or at delivery.
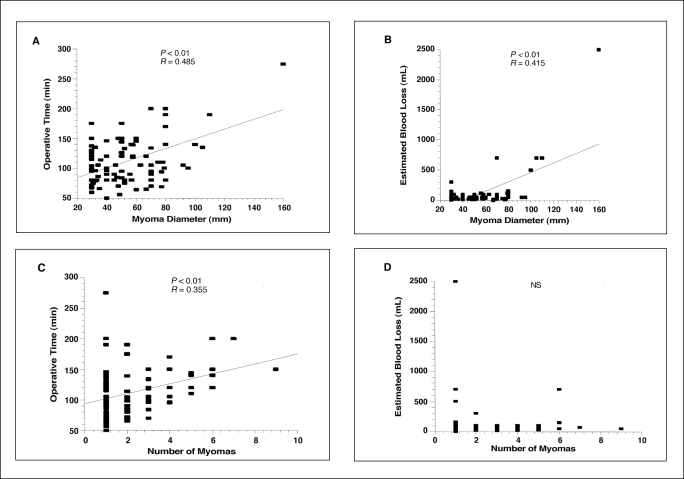
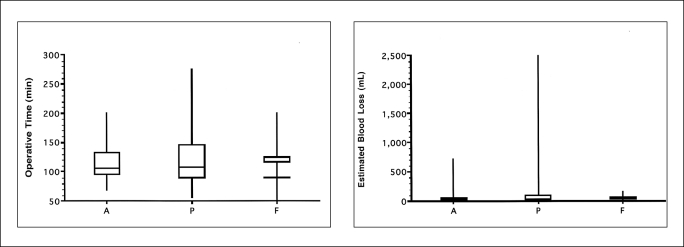
Regarding the correlation calculated between operative time, estimated blood loss, the dominant myoma diameter, and the number of myomas, the results can be summarized as follows: Operative time was significantly positively correlated (P<0.01) with the dominant myoma diameter and the number of myomas; estimated blood loss was significantly positively correlated with myoma size (P<0.01), but no correlation existed with the number of myomas (Figure 3). Operative time and estimated blood loss were not significantly related to the location (anterior, posterior, fundus, and in the broad ligament) of the myoma (Figure 4).
Figure 3.
Relationship between dominant myoma diameter and operative time and estimated blood loss (A, B) and between the number of myoma and operative time and estimated blood loss (C, D).
Figure 4.
The operative time and estimated blood loss among the dominant myoma locations (A: anterior, P: posterior, F: fundus).
In this study, the risk of perioperative heavy bleeding (> 500 mL) did not correlate with the number of myomectomies, or the location of the dominant myoma that was removed. The risk of heavy bleeding is a significant correlate with largest myoma size. When the myoma is larger than 10 cm, the blood loss and operative time are estimated to be greater. A limit of 10 cm has been proposed as the maximum size for LM.
DISCUSSION
Among the laparoscopic operations carried out in the field of gynecology, LM is fraught with problems. Solutions, including those involving the enucleation of the myoma, suturing of the myometrium, and the recovery of solid myomas from the body of the uterus, and the indications for and limitations of LM have not been clearly defined. The points of prime importance in performing effective laparoscopic enucleation of myomas from various sites and depths in the uterus, ensuring that the suturing of the myometrium is complete, and that the myoma is fully extracted, are where the operator stands and where the point of the trocar is inserted. Our method, in which the operator stands on the left side of the patient and controls a left upper-quadrant trocar (10 mm) and a left lower-quadrant trocar (5 mm) by his or her right and left hands, respectively, seems to be appropriate for achieving our purpose in LM. Because the forceps are manipulated through an upper-quadrant trocar by the operator's right hand, traction of the myoma and suturing of the myometrium using a myoma screw are facilitated. Koh et al3 also has stated that, when laparoscopic suturing is performed, the use of ipsilateral upper and lower trocars permits the prompt and fatigue-free operation of forceps, unlike the use of right and left trocars by crossing one of the operator's hands with the scope. When LM is performed, the use of vasopressin is effective, because bleeding from the wound surface during enucleation obstructs the view of the operative field, making enucleation and suturing difficult. The myoma is turned a pale color by the vasoconstrictor action that immediately follows the local administration of vasopressin between the myometrium and the capsule of the myoma.4 This effect persists for 20 to 30 minutes. The capsule of the nucleus should be incised and detached on the surface from the myometrium to achieve a successful myomectomy, and for this purpose, it is important to draw the capsule of the myoma perpendicularly from the myometrium. Because the traction can be performed only through the insertion point of a trocar in LM, the procedure is extremely limited compared with techniques using laparotomy in which the traction of a myoma can be performed in any direction. The apical chip of the Uterine Manipulator™ can move 90 degrees rearwards and 120 degrees forwards. When a myoma nucleus is present in the anterior wall, the uterus should be kept in a retroflexed position; and when it is in the posterior wall, the uterus should be kept in an anteflexed position. The myoma nuclei can then be drawn perpendicularly to the long axis of the uterus through a left upper quadrant trocar by using a myoma screw. Enucleation of myoma from any site, including the anterior wall, the posterior wall, the fundus and the broad ligament, can be carried out using this procedure. Rossetti et al5 made an articulated myoma drill whose tip can be bent at a right angle to design the traction method for myoma nuclei, but our methods in which the direction of traction by a myoma screw is fixed while the uterine position is adjusted by a Uterine Manipulator™ appears easier than theirs.
Either a longitudinal or a transverse hysterotomy of the uterine wall can be performed for myomectomy,6 but we perform only the latter, irrespective of the site of the myoma, because suturing toward the long axis of the uterus in our method, in which a needle holder is operated through a left upper quadrant trocar, is easy. Igarashi7 has proposed that a transverse incision of the uterus and suturing of the myometrium toward the long axis of the uterus should be performed, on the basis of the hemodynamics of the myometrium, in which the arcuate artery from the ascending branch of the uterine artery crosses the uterine body and the radial artery branches from the arcuate artery to the uterine endometrium. The suturing of the myometrium in LM requires the greatest degree of skill, but it can be performed smoothly and easily by our method, because the needle holder can be controlled by the operator's right hand through the left upper quadrant trocar, which has extensive working space. Laparoscopic suturing can be performed similarly to suturing in laparotomy if the operator is familiar with the suturing technique, and we usually suture the myometrium with an average of 9 stitches, although more than 20 are necessary in some cases. Myomectomy aims to preserve fertility, and the prevention of postoperative adhesions that reduce fertility is important. The baseball suture, in which the incised layer is embedded, is adequate for suturing between superficial myometrium and serosa. It is difficult to perform a laparoscopic baseball suture with a common curved needle, but it can be performed easily and quickly with the Endostitch™. The Endostitch™ is a suturing device to which the mechanism of a hand sewing machine is applied and in which a straight, certainly threaded needle shuttles to and fro through an apical jaw. Thus, the baseball suture can be easily performed without changing hands. Furthermore, the wound surface is coated with fibrin glue spray to prevent postoperative adhesion. Fibrin glue is effective not only as a hemostatic, but also in preventing adhesion, because the wound surface is covered with a gelatinous capsule for about 1 week until the completion of primary healing in vivo. The prophylactic effect of the glue against postoperative adhesion has been confirmed by SLL in the enucleation of ovarian endometrioma.8
The extraction of enucleated myoma from the uterine body is one of the primary limiting factors in LM, and various extraction methods have been designed. We have performed internal crushing and recovery by colpotomy, and when the diameter of a myoma nucleus is 5 cm or over, the recovery time is extremely long. An electric morcellator has been adopted, so that a large myoma can also be extracted through a trocar site.
As the result of improvements in these surgical procedures and of the development of new devices, the indications for LM have been significantly broadened. Of 89 patients who underwent LM from 1998 to 1999, none were converted to laparotomy. In a study on the amount of bleeding and the time required for surgery, a positive correlation between the size of the myoma and both the time required for surgery and the amount of bleeding were observed, but the number of myomas showed a positive correlation only with the duration of the operation. If the diameter of a myoma is ≥ 10 cm, the time required for suturing and extraction will be significantly prolonged, and the risk of loosing more than 500 mL of blood will be increased. Dubuisson et al9 divided the patients undergoing LM into 2 groups, one completing LM alone and the other requiring postoperative laparotomy. They studied the indications for LM and reported that LM was indicated in 1 or 2 myoma nuclei of 8 cm or less in diameter. In addition, Maris et al10 reported that the requirements for the safe performance of LM are a myoma size of 7 cm or less with no more than 5 total. The present study revealed that a limiting factor of LM is the size of the myoma nucleus and that LM seems to be indicated in myomas of 10 cm or less in diameter. As for the number of myomas, specific criteria have not been established; but if the maximum diameter of the myomas is 5 cm or less, LM seems to be indicated for up to 5 myomas. Furthermore, Dubuisson et al9 reported that, if myomas are present in the anterior wall, the risk of needing laparotomy is increased, but the present study showed that the sites of the myomas do not affect the time required for operation or the amount of bleeding and indicated that our method can be applied to myomas at any site. In addition, preoperative GnRHa administration reduces the size of the myoma and decreases the blood flow in the uterine artery, so that the indications for LM are extended, the operative procedure becomes easier, and the amount of bleeding is decreased.11 In this study, 36 patients (63.2%) were given GnRHa preoperatively, and GnRHa's usefulness in LM was confirmed.
LM has the common merits of laparoscopic operations, such as less operative stress and a shorter postoperative recovery than that of laparotomy. In addition, it has also been reported that the preservation of fertility after LM is superior to that after laparotomy because less postoperative adhesions occur in LM than in laparotomy.12,13 In this study, the postoperative hospitalization period was approximately 2 days, and 10 (29.4%) of 34 infertile patients became pregnant. Four patients who had already delivered vaginally had intramural myomas, and only 1 patient with a submucosal myoma required a Cesarean delivery. Rupture of the uterus14 in pregnancy and at delivery as a result of the failure of the myometrial suture, which was first reported soon after the introduction of LM, seems to have been overcome by ensuring complication of the suturing. Unlike laparotomy, LM has the shortcoming of difficulty in diagnosing small myomas deep in the myometrium by palpation of the uterine body, so that these may be missed in LM. It has also been reported that the recurrence rate of myomas is higher in LM than in laparotomy.15 When LM is performed in multiple myomas, it is important to know precisely the sites of the myoma by preoperative diagnostic imaging techniques, such as MRI.
LM is being established as one of the basic laparoscopic operative techniques in the field of gynecology, and further study of its indications and long-term prognosis is expected.
Contributor Information
Hiroyuki Takeuchi, Department of Obstetrics and Gynecology, Juntendo University School of Medicine, Tokyo, Japan..
Ryohei Kuwatsuru, Department of Radiology, Juntendo University School of Medicine, Tokyo, Japan..
References:
- 1. Dubuisson JB, Mandelbrot LM, Lecuru F, et al. Myomectomy by laparoscopy: a preliminary report of 43 cases. Fertil Steril. 1991; 56: 827–830 [DOI] [PubMed] [Google Scholar]
- 2. Nezhat C, Nezhat F, Sifen SL, et al. Laparoscopic myomectomy. Int J Fertil. 1991; 36: 275–280 [PubMed] [Google Scholar]
- 3. Koh CH, Janik GM. Laparoscopic microsurgical tubal anastomosis-difficulties and pitfalls. In: Corfman RS, Diamond MP, DeCherney, eds. Complications of laparoscopy and hysteroscopy, 2nd ed. Massachusetts: Blackwell Science; 1997: 107–113 [Google Scholar]
- 4. Seinera P, Arisio R, Decko A, et al. Laparoscopic myomectomy: indications, surgical technique and complications. Hum Reprod. 1997; 12: 1927–1930 [DOI] [PubMed] [Google Scholar]
- 5. Rossetti A, Paccosi M, Sizzi O, et al. Dilute ornithine vasopressin and myoma drill for laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1999; 6: 189–193 [DOI] [PubMed] [Google Scholar]
- 6. Dubuisson JB, Chapron C, Mouly M, et al. Laparoscopic myomectomy. Gynecol Endosc. 1993; 2: 171–173 [Google Scholar]
- 7. Igarashi M. Value of myomectomy in the treatment of infertility. Fertil Steril. 1993; 59( 6): 1331–1332 [DOI] [PubMed] [Google Scholar]
- 8. Takeuchi H, Hashimoto M, Masanori A, et al. Reduction of post operative adhesions with fibrin glue after laparoscopic excision of large ovarian endometrioma. J Am Assoc Gynecol Laparosc. 1996; 3: 575–578 [DOI] [PubMed] [Google Scholar]
- 9. Dubuisson JB, Chapron C, Levy L. Difficulties and complications of laparoscopic myomectomy. J Gynecol Surg. 1996; 12: 159–165 [Google Scholar]
- 10. Maris V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: A prospective randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996; 174: 654–658 [DOI] [PubMed] [Google Scholar]
- 11. Zullo F, Pellicano M, Carlo CD, et al. Ultrasonographic prediction of the efficacy of GnRH agonist therapy before laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1998; 5: 361–366 [DOI] [PubMed] [Google Scholar]
- 12. Dubisson JB, Fauconnier A, Chapron C, et al. Second look after laparoscopic myomectomy. Hum Reprod. 1998; 13: 2102–2106 [DOI] [PubMed] [Google Scholar]
- 13. Bulletti C, Polli V, Negrini V, Giacomucci E, et al. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996; 3: 533–536 [DOI] [PubMed] [Google Scholar]
- 14. Harris WJ. Uterine dehiscence following laparoscopic myomectomy. Obstet Gynecol. 1992; 80: 545–546 [PubMed] [Google Scholar]
- 15. Nezhat FR, Roemisch M, Nezhat CH, et al. Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1998; 5( 3): 237–240 [DOI] [PubMed] [Google Scholar]