Abstract
The major apoprotein of high density lipoprotein is apolipoprotein A-I (ApoA-I). In addition to being a structural component of this class of lipoproteins, ApoA-I also has a physiologic role as an activator of lecithin-cholesterol acyl transferase, an enzyme important in the metabolism of all lipoproteins.
To measure ApoA-I content in human plasma, to assess its immunologic activity in hyperlipoproteinemia, and to carry out certain structural studies of high density lipoproteins, we have developed a double antibody radioimmunoassay. ApoA-I, isolated by gel filtration, was used to produce monospecific antisera. ApoA-I was iodinated by chloramine-T and the resulting [125I]-ApoA-I was purified by gel filtration. > 85% of [125I]-ApoA-I was precipitated by antibody, and 90% of bound [125I]ApoA-I was displaced by “cold” ApoA-I. Other lipoproteins and apoproteins did not react.
Plasma and high density lipoprotein from normals and subjects with hyperlipoproteinemia displaced counts in parallel with ApoA-I, suggesting that the same antigenic determinants were reacting with antibody on lipid-free and lipid-associated ApoA-I. However, less than 5% of ApoA-I of high density lipoprotein reacted in the assay. Removal of the lipid by extraction increased the reactivity of ApoA-I in high density lipoprotein 15-20-fold; thus more than 95% of the ApoA-I molecules in “intact” high density lipoprotein are unreactive with antibody. Normal and hyperlipoproteinemic plasma and high density lipoproteins isolated from the same subjects continued to display parallelism with ApoA-I standard after lipid extraction, suggesting that ApoA-I of normal and hyperliproteinemic subjects are immunologically identical.
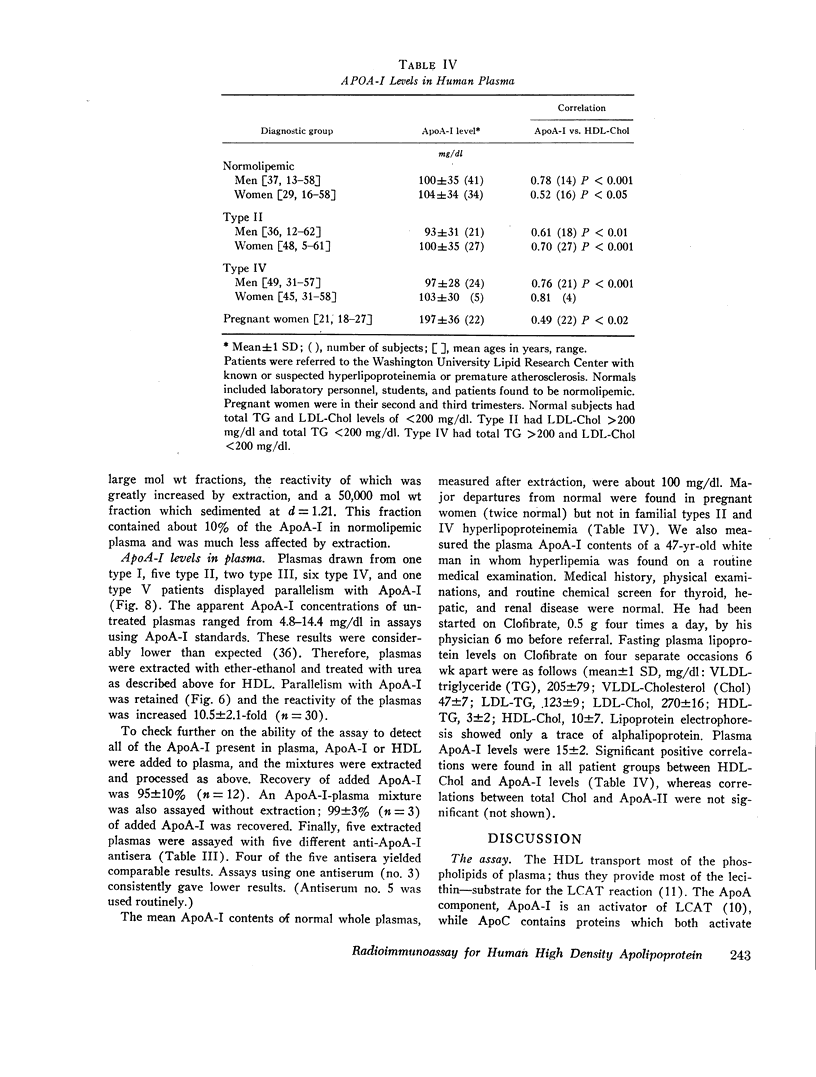
About 90% of ApoA-I was in the d 1.063-1.21 fractions of normal plasma, trace quantities were found in the lipoproteins of d < 1.063, and the rest (about 10%) was in the d > 1.21 fraction. Normal plasma levels, assessed in extracted plasmas with a precision of 8%, were 100±35 mg/dl. Levels were normal in small groups of subjects with types II and IV hyperlipoproteinemia and high in pregnancy. However, larger population studies need to be performed to determine the distribution of ApoA-I levels in the various hyperlipoproteinemias.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alaupovic P., Lee D. M., McConathy W. J. Studies on the composition and structure of plasma lipoproteins. Distribution of lipoprotein families in major density classes of normal human plasma lipoproteins. Biochim Biophys Acta. 1972 Apr 18;260(4):689–707. [PubMed] [Google Scholar]
- BLUMBERG B. S., BERNANKE D., ALLISON A. C. A human lipoprotein polymorphism. J Clin Invest. 1962 Oct;41:1936–1944. doi: 10.1172/JCI104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H. N., Jackson R. L., Gotto A. M., Jr Isolation and characterization of the cyanogen bromide fragments from the high-density apolipoprotein glutamine I. Biochemistry. 1973 Sep 25;12(20):3866–3871. doi: 10.1021/bi00744a011. [DOI] [PubMed] [Google Scholar]
- Borut T. C., Aladjem F. Immunochemical heterogeneity of human high density serum lipoproteins. Immunochemistry. 1971 Sep;8(9):851–863. doi: 10.1016/0019-2791(71)90451-4. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Baginsky M. L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972 Jan 31;46(2):375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eaton R. P., Kipnis D. M. Radioimmunoassay of beta lipoprotein-protein of rat serum. J Clin Invest. 1969 Aug;48(8):1387–1396. doi: 10.1172/JCI106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehnholm C., Garoff H., Simons K., Aro H. Purification and quantitation of the human plasma lipoprotein carrying the Lp(a) antigen. Biochim Biophys Acta. 1971 May 25;236(2):431–439. doi: 10.1016/0005-2795(71)90223-6. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Lim C. T., Scanu A. M. A protein component of serum high density lipoprotein with CO-factor activity against purified lipoprotein lipase. Biochem Biophys Res Commun. 1970 Jun 5;39(5):889–894. doi: 10.1016/0006-291x(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Nichols A. V., Gong E. L., Levy R. I., Lux S. Electron microscopic study on reassembly of plasma high density apoprotein with various lipids. Biochim Biophys Acta. 1971 Nov 5;248(2):381–386. doi: 10.1016/0005-2760(71)90026-9. [DOI] [PubMed] [Google Scholar]
- Forte T., Norum K. R., Glomset J. A., Nichols A. V. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: structure of low and high density lipoproteins as revealed by elctron microscopy. J Clin Invest. 1971 May;50(5):1141–1148. doi: 10.1172/JCI106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Jan 26;276(4):215–contd. doi: 10.1056/NEJM196701262760406. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan D., Bradford R. H., Alaupovic P., McConathy W. J. Differential activation of lipoprotein lipase from human post-heparin plasma, milk and adipose tissue by polypeptides of human serum Apolipoprotein C. FEBS Lett. 1971 Jun 24;15(3):205–208. doi: 10.1016/0014-5793(71)80312-5. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Havel R. J., Shore V. G., Shore B., Bier D. M. Role of specific glycopeptides of human serum lipoproteins in the activation of lipoprotein lipase. Circ Res. 1970 Oct;27(4):595–600. doi: 10.1161/01.res.27.4.595. [DOI] [PubMed] [Google Scholar]
- LEVY R. I., FREDRICKSON D. S. HETEROGENEITY OF PLASMA HIGH DENSITY LIPOPROTEINS. J Clin Invest. 1965 Mar;44:426–441. doi: 10.1172/JCI105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laggner P., Kratky O., Kostner G., Sattler J., Holasek A. Small angle X-ray scattering of LpA, the major lipoprotein family of human plasma high density lipoprotein HDL(3). FEBS Lett. 1972 Oct 15;27(1):53–57. doi: 10.1016/0014-5793(72)80408-3. [DOI] [PubMed] [Google Scholar]
- Laggner P., Müller K., Kratky O., Kostner G., Holasek A. Studies on the structure of lipoprotein A of human high density lipoprotein HDL3: the spherically averaged electron density distribution. FEBS Lett. 1973 Jun 15;33(1):77–80. doi: 10.1016/0014-5793(73)80163-2. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Brewer H. B., Jr Isolation and characterization of apoLp-Gln-II (apoA-II), a plasma high density apolipoprotein containing two identical polypeptide chains. J Biol Chem. 1972 Dec 10;247(23):7510–7518. [PubMed] [Google Scholar]
- Lux S. E., Levy R. I., Gotto A. M., Fredrickson D. S. Studies on the protein defect in Tangier disease. Isolation and characterization of an abnormal high density lipoprotein. J Clin Invest. 1972 Oct;51(10):2505–2519. doi: 10.1172/JCI107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley A. R., Jr, Niswender G. D., Rebar R. W. Principles for the assessment of the reliability of radioimmunoassay methods (precision, accuracy, sensitivity, specificity). Acta Endocrinol Suppl (Copenh) 1969;142:163–184. doi: 10.1530/acta.0.062s163. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Wisdom C. Serum lipoproteins structure and function. Annu Rev Biochem. 1972;41:703–730. doi: 10.1146/annurev.bi.41.070172.003415. [DOI] [PubMed] [Google Scholar]
- Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966 Mar;7(2):295–306. [PubMed] [Google Scholar]
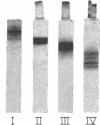
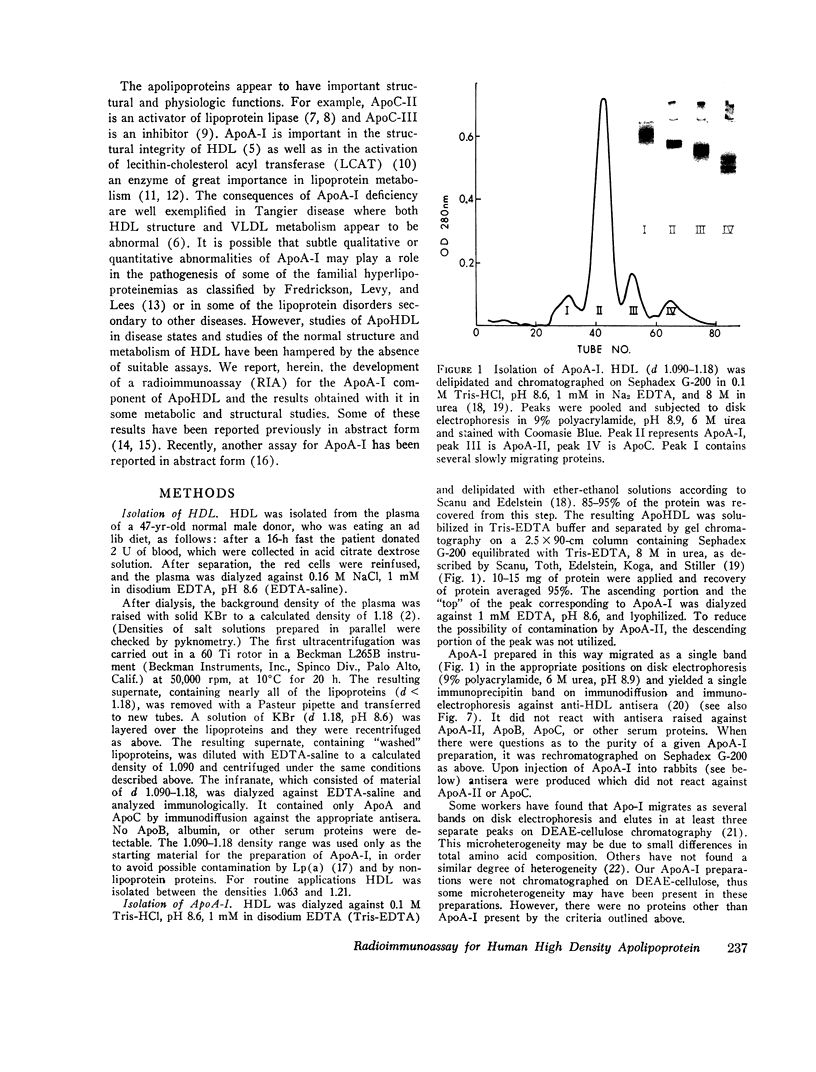
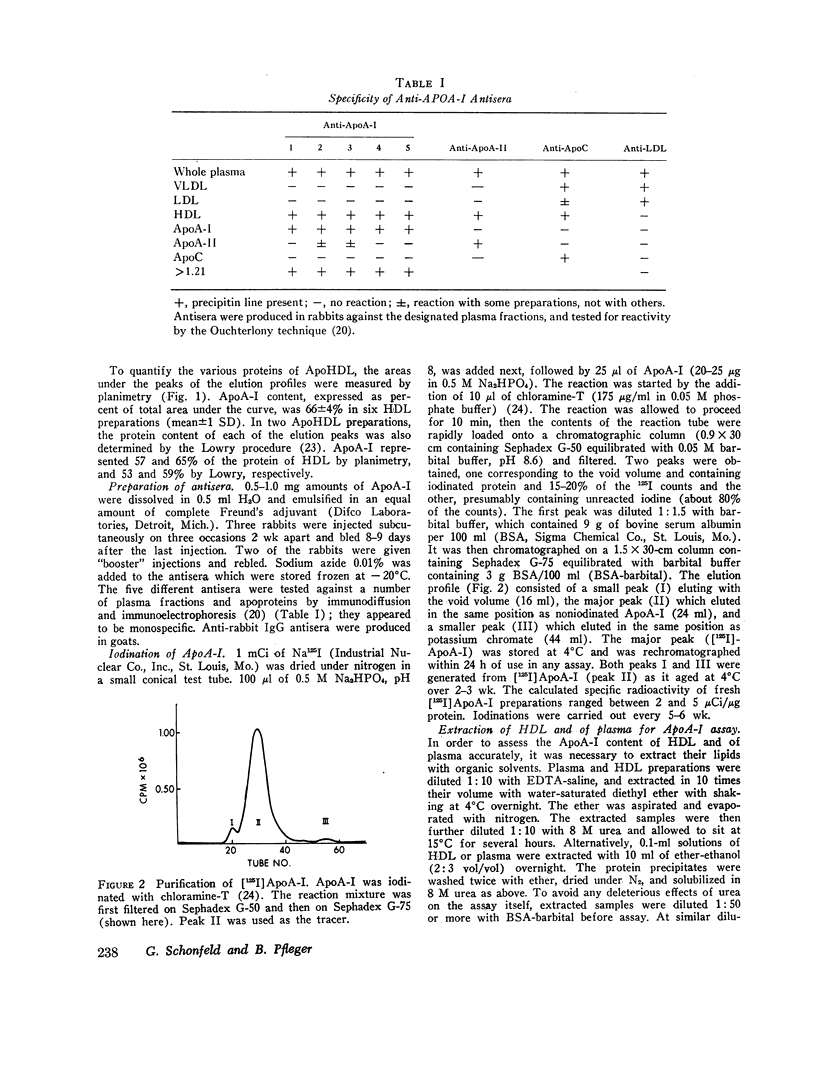
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Schonfeld G., Lees R. S., George P. K., Pfleger B. Assay of total plasma apolipoprotein B concentration in human subjects. J Clin Invest. 1974 May;53(5):1458–1467. doi: 10.1172/JCI107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Adams G. H. Circulating lipoproteins. Annu Rev Biochem. 1969;38:113–136. doi: 10.1146/annurev.bi.38.070169.000553. [DOI] [PubMed] [Google Scholar]
- Shore B., Shore V. Heterogeneity in protein subunits of human serum high-density lipoproteins. Biochemistry. 1968 Aug;7(8):2773–2777. doi: 10.1021/bi00848a011. [DOI] [PubMed] [Google Scholar]