Abstract
Introduction:
The management of pediatric empyema remains controversial. We contend that early thoracoscopic intervention results in shorter hospital stays, decreased morbidity, and superior outcomes. We propose an algorithm using early image-guided thoracoscopy as an effective treatment for pediatric empyema.
Methods:
Consecutive pediatric empyemas treated from November 1997 to April 2001 using a prospective management algorithm were reviewed. Demographic data, days to diagnosis, days to surgery, length of stay, chest tube days, complications, and follow-up were recorded.
Results:
Twenty-two children with 24 empyemas were treated using this algorithm. Their mean age was 49 months. Mean days to diagnosis was 11 and from diagnosis to surgery was 3. Imaging included chest radiography (CXR) in all, ultrasound in 17 (77%), and computed tomography (CT) scan in 13 (59%). One thoracoscopy was converted to a mini-thoracotomy because of difficulty with ventilation. Chest tube removal averaged 3 days with an average length of stay of 13 days. One patient required a second thoracoscopy for recurrent empyema, and 1 patient developed a contralateral empyema. No other complications or deaths occurred. Follow-up in 19 of 22 (86%) children at 5 months revealed no recurrences or mortality.
Conclusions:
This treatment algorithm, using early image-guided thoracoscopy, is a safe and effective means of managing pediatric empyema, while shortening hospital stay and avoiding the morbidity of thoracotomy.
Keywords: Empyema, VATS, Pediatric thoracoscopy, Algorithm
INTRODUCTION
A consensus regarding the appropriate treatment, as well as the timing of treatment, for children with empyema continues to elude us. Treatment options are varied, ranging from antibiotics, instillation of fibrinolytics, thoracentesis, thoracoscopy, to open thoracotomy. Additionally, the threshold for surgical intervention is ill defined. Video-assisted thoracic surgery (VATS) offers the distinct advantage of minimizing operative morbidity, and as technology and surgical skill have improved, so has its promise in regards to the treatment of empyema in children. We have previously published our results of the use of early VATS in managing pediatric empyemas and have formulated an algorithm.1 Therefore, we reviewed our experience using this algorithm to manage pediatric empyema in a prospective fashion in nonselected consecutive patients.
METHODS
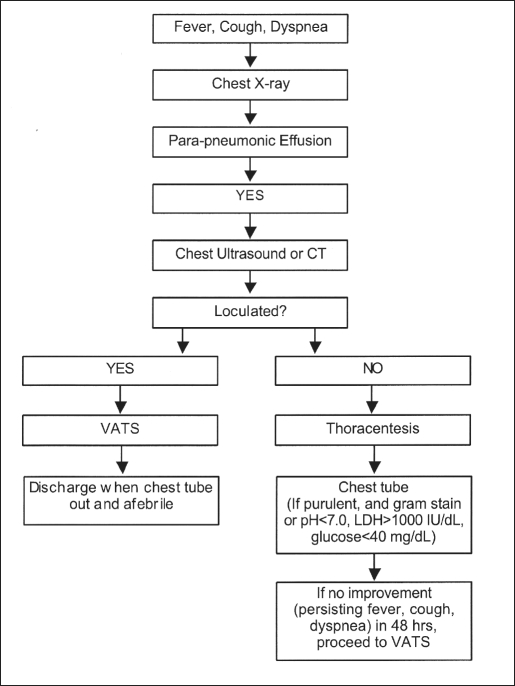
We reviewed the records of 22 consecutive children who were diagnosed with empyema from November 1997 to April 2001. All patients were managed with our algorithm (Figure 1).1 The presumptive diagnosis of empyema was made preoperatively based on clinical examination, chest radiography, and imaging using ultrasound or computed tomography (CT).
Figure 1.
Treatment algorithm for pediatric empyema.
If a free-flowing fluid collection was found, thoracentesis was undertaken. If an empyema was present (ie, frank purulence, positive gram stain, pH < 7, LDH > 1000 IU/dL, glucose < 40 mg/dL), a chest tube was placed. However, if a loculated fluid collection was seen on imaging studies, the patient underwent VATS for the empyema without any other preoperative interventions. Chest tubes were removed when the patient was clinically improving and drainage was less than 50 mL/day.
Our technique for VATS in managing pediatric empyema in brief is carried out with either a single-lumen endotracheal tube with selective bronchial intubation or a double-lumen endotracheal tube. The patient is placed in a lateral decubitus position with trocar placement dictated by the location of the empyema. One trocar is placed for the telescope and 2 subsequent incisions are made under direct visualization. These are placed in such a manner that they can be included in a formal thoracotomy incision if necessary. A 5- or 10-mm telescope is utilized depending on the size of the patient. All purulent and fibrinous material is suctioned, and the visceral and parietal pleura are completely débrided. If circumstances preclude effective débridement (ie, excessive bleeding, poor visualization, inability to tolerate single lung ventilation), the procedure is converted to a mini-thoracotomy. All specimens are sent for culture and pathologic analysis. The pleural cavity is then thoroughly irrigated and a chest tube placed.
The majority of children were transferred to the pediatric floor where chest tubes were removed when the lung was fully expanded and drainage was less than 50 mL/day. Intravenous antibiotics were started preoperatively on all patients and continued postoperatively until the patient was afebrile. All patients were discharged home on oral antibiotics and follow-up included a chest x-ray. Demographic data, days to diagnosis, days to surgery, length of hospital stay, postoperative chest tube days, complications, and follow-up results were recorded and are displayed where appropriate as means ± standard deviation.
RESULTS
Twenty-two children with 24 empyemas were treated using this algorithm. The mean age was 49 months (8 to 151 months). Fourteen were males and 8 were females. Common presenting symptoms were fever, cough, and tachypnea. The average number of days from onset of symptoms to diagnosis was 11±7 days. The average number of days from diagnosis to surgery was 3±3 days. Average length of stay was 13±5 days.
Thoracentesis was performed in 9 children preoperatively (3 at outside institutions) with 6 positive cultures (Streptococcus pneumoniae, 3; Streptococcus pyogenes, 3). The 6 children who underwent thoracentesis at our institution had them performed by a pediatric intensivist prior to our consultation. Subsequent workup in all but one of these cases revealed loculated fluid collections and therefore only 1 chest tube was placed. This child went on to develop a loculated effusion that failed chest tube drainage, and the child subsequently underwent successful VATS. Two chest tubes were placed preoperatively (one at an outside institution without a preceding thoracentesis).
All children were imaged with chest x-rays; ultrasound was used in 17 (77%) and CT in 13 (59%). In 2 children, loculated fluid was seen on initial lateral and decubitus chest x-rays, and no further studies were obtained prior to surgery. In 7 children, CT was equivocal for loculations, but in all 7 cases, loculated fluid collections were confirmed by chest ultrasound examination.
All 22 children underwent VATS once loculated fluid collections were identified radiographically. One child who has an immunodeficiency required a second VATS for a second empyema, which developed 1 month later, with successful resolution of her empyema. Another child with streptococcal sepsis and acute renal failure developed a contralateral empyema. Both these children were effectively treated by a second VATS decortication. One child had a residual left upper lobe loculation, which resolved with the placement of a CT-guided percutaneous drain during the same hospitalization. It was removed 3 days later, and the patient was discharged uneventfully the following day. One child underwent mini-thoracotomy secondary to difficulty with ventilation and was found to have an abscess with a necrotic right upper lobe that required resection. Four operative cultures grew bacteria (Streptococcus pyogenes, 1; Streptococcus pneumoniae, 3). No deaths occurred. Chest tubes were removed in 3±2 days.
Nineteen children presented for follow-up (86%) at a mean of 5 months postoperatively. All children received chest x-rays, which uniformly showed remarkable improvement. One displayed evidence of a pneumatocele at 1 month. All 19 were clinically stable and free from symptoms at the follow-up office visit.
DISCUSSION
Most empyemas arise as a result of preceding pneumonia but less than 1% of pneumoniae result in an empyema.2 All of our patients’ empyemas resulted from a parapneumonic effusion. Classically, the natural progression of an empyema consists of 3 stages.3 The first is the “exudative” or “acute” phase. The pleural accumulation is a thin, free-flowing, purulent fluid. A thickening of the pleural exudate and development of a peel with resultant loculations characterize the ‘fibrinopurulent’ stage. The final stage is one of “organization,” where the loose peel becomes more tenacious with fibrosis and scar formation.
Traditionally, administration of prolonged intravenous antibiotics with or without drainage (thoracentesis or chest tube) has been the treatment algorithm, and only when progression to the final organizing stage of empyema has occurred is open thoracotomy undertaken. The perceived associated morbidity of an open thoracotomy is often the reason for prolonged nonoperative management of empyemas. Surgeons are increasingly advocating early rather than late surgical drainage to reduce length of hospital stay and need for long-term antibiotics. The increasing utilization of thoracoscopy and rapidly improving technology has compelled many to replace an open decortication with a thoracoscopic approach. Several authors have presented their successful experiences with thoracoscopic drainage of empyemas in children.1, 4–14
Our algorithm is based on the presence or absence of radiological signs of loculations rather than operating within a specific time frame. The majority of these children presented to our service having already progressed to the fibrinopurulent stage. The pleural space may be evaluated by ultrasound or CT scan. In our experience, chest sonography is the initial imaging modality of choice, and it detected loculations in 100% of the cases in which it was used. Several groups have found it to be better than CT in evaluating pleural fluid collections.15,16 A chest CT scan may also be indicated to evaluate the lung parenchyma if clinical or radiological suspicion of a lung abscess exists.
A mean length of hospital stay of up to 27 days has been reported by others17 while our experience was 13 days. Chest tubes were removed an average of 3 days after surgery, avoiding the morbidity of prolonged indwelling chest tubes. Follow-up was available in 19 children, and none developed recurrences of their empyemas, aside from 1 patient with a relatively normal chest x-ray but who had an immunodeficiency and developed a recurrent empyema 1 month following discharge from the hospital.
CONCLUSION
Few would argue that the ideal treatment of an empyema would involve minimal morbidity with a speedy recovery and very low recurrence rate. Our experience using an image-guided treatment algorithm accomplishes this via a simple, safe, and effective process. It joins the growing body of evidence supporting VATS in the treatment of pediatric empyema.
Footnotes
Presented at the 11th Annual Congress for Endosurgery in Children, Genova, Italy, May 2-4, 2002.
Contributor Information
Jason Knudtson, Department of Surgery, University of Kansas School of Medicine–Wichita, Wichita, Kansas, USA..
Harsh Grewal, Department of Surgery, Section of Pediatric Surgery, Temple University, Philadelphia, Pennsylvania, USA..
References:
- 1. Grewal H, Jackson RJ, Wagner CW, Smith SD. Early video-assisted thoracic surgery in the management of empyema. Pediatrics. 1999; 103 ( 5): e63. [DOI] [PubMed] [Google Scholar]
- 2. Chonmaitree T, Powell KR. Parapneumonic pleural effusions and empyema in children. Clin Pediatr. 1983; 22: 414– 419 [DOI] [PubMed] [Google Scholar]
- 3. Tuggle DW. Acquired pulmonary and pleural disorders. In: Ashcraft KW, ed. Pediatric Surgery. Philadelphia, PA: WB Saunders Co; 2000: 287– 289 [Google Scholar]
- 4. Kern JA, Rodgers BM. Thoracoscopy in the management of empyema in children. J Pediatr Surg. 1993; 28: 1128– 1132 [DOI] [PubMed] [Google Scholar]
- 5. Silen ML, Weber TR. Thoracoscopic debridement of loculated empyema thoracis in children. Ann Thorac Surg. 1995; 59 1166– 1168 [DOI] [PubMed] [Google Scholar]
- 6. Stovroff M, Teague G, Heiss KF. et al. Thoracoscopy in the management of pediatric empyema. J Pediatr Surg. 1995; 30: 1211– 1215 [DOI] [PubMed] [Google Scholar]
- 7. Davidoff AM, Hebra A, Kerr J, Stafford PW. Thoracoscopic management of empyema in children. J Laparoendosc Surg. 1996; 6: S51– S54 [PubMed] [Google Scholar]
- 8. Klena JW, Cameron BH, Langer JC. et al. Timing of video-assisted thoracoscopic debridement for pediatric empyema. J Am Coll Surg. 1998; 187: 404– 408 [DOI] [PubMed] [Google Scholar]
- 9. Gandhi RR, Stringel G. Video-assisted thoracoscopic surgery in the management of pediatric empyema. J Soc Laparoendosc Surg. 1997; 1: 251– 253 [PMC free article] [PubMed] [Google Scholar]
- 10. Patton RM, Abrams RS, Gauderer MW. Is thoracoscopically aided pleural debridement advantageous in children? Am Surg. 1999; 3: 69– 72 [PubMed] [Google Scholar]
- 11. Merry CM, Bufo AJ, Shah RS. et al. Early definitive intervention by thoracoscopy in pediatric empyema. J Pediatr Surg. 1999; 34: 178– 181 [DOI] [PubMed] [Google Scholar]
- 12. Kercher KW, Attorri RJ, Hoover JD, Morton D. Thoracoscopic decortication as first-line therapy for pediatric parapneumonic empyema. A case series. Chest. 2000; 118: 24– 27 [DOI] [PubMed] [Google Scholar]
- 13. McGahren ED. Use of thoracoscopy for treatment of empyema in children. Ped Endosurg Innov Tech. 2001; 5: 117– 125 [Google Scholar]
- 14. Rothenberg SS, Chang JHT. Thoracoscopic decortication in infants and children. Surg Endosc. 1997; 11: 93– 94 [DOI] [PubMed] [Google Scholar]
- 15. Park CS, Chung WM, Lim MK. et al. Transcatheter instillation of urokinase into loculated pleural effusion: analysis of treatment effect. AJR Am J Roentgenol. 1996; 167: 649– 652 [DOI] [PubMed] [Google Scholar]
- 16. Donnelly LF, Klosterman LA. CT appearance of para-pneumonic effusions in children: findings are not specific for empyema. AJR Am J Roentgenol. 1997; 169: 179– 182 [DOI] [PubMed] [Google Scholar]
- 17. Chan W, Keyser-Gauvin E, Davis GM, et al. Empyema thoracis in children: a 25-year review of the Montreal Children's Hospital experience. J Pediatr Surg. 1997; 32: 870– 872 [DOI] [PubMed] [Google Scholar]