Abstract
Background and Objectives:
Gastroesophageal reflux disease (GERD) is commonly associated with morbid obesity (MO). Antireflux surgery has a higher failure rate in MO and addresses only one of the comorbidities present. This paper reviews the results of laparoscopic Rouxen-Y gastric bypass (LRYGBP) performed for recalcitrant GERD in MO.
Methods:
Patients with recalcitrant GERD and a body mass index (BMI)>35 undergoing LRYGBP were included. LRYGB included crural repair, creation of a small gastric pouch (30 mL), and intestinal bypass (150 to 180 cm). All patients were followed in clinic and by telephone.
Results:
From February 1999 to April 2001, 57 patients (51 F, 6 M) with a mean age of 43 (range, 22 to 67) and a median BMI of 43 underwent LRYGBP. Hiatal hernia or esophagitis, or both, were present in 48, Barrett's in 2. LRYGBP was possible in 52 patients; 5 required open conversion. The median hospital stay was 3 days. Complications included 1 leak, 1 pulmonary emboli, 2 reoperations for internal roux limb hernia, and 7 gastrojejunal strictures. At a mean follow-up of 18 months (range, 3 to 30), all patients report improvement or no symptoms of GERD and a mean weight loss of 40 kg (range, 16 to 70). Quality of life scores (SF-36) were above national norms for physical and mental components (median 55, norms=50). GERD-health related quality of life median score was <1 (scale, 0 to 45, 0=asymptomatic, 45=worse).
Conclusion:
LRYGBP was effective for recalcitrant GERD in MO. LRYGBP also led to weight loss and improvement in other comorbidites. Surgeons with minimally invasive expertise should consider LRYGBP for treatment of GERD in the morbidly obese.
Keywords: Gastric bypass, Gastroesophagel reflux disease, Morbid obesity
INTRODUCTION
Gastroesophageal reflux disease (GERD) is one of the most frequently occurring benign functional disorders in Western industrial countries.1 The effectiveness of laparoscopic antireflux surgery for recalcitrant GERD has been clearly demonstrated in several series. Good to excellent patient satisfaction scores have been reported in approximately 90% of patients.2,3 These laparoscopic results, in combination with a shorter hospital stay and a more rapid return to normal activities, have promoted the emergence of minimally invasive antireflux surgery as the method of choice for the operative management of GERD.4
Antireflux surgery has a higher failure rate in MO, which is in direct relation to high body mass index.5 The increased intraabdominal pressure and the morbid obesity-related comorbidities lead to a higher failure rate of the standard antireflux procedures in this group of patients. Over the past 40 years, surgery has become the most effective long-term treatment for morbid obesity.6 The National Institutes of Health during their Consensus Development Conference on Gastrointestinal Surgery for Morbid Obesity in 1991 recognized the role of bariatric surgery in the treatment of highly selected, well-informed, motivated patients who are acceptable operative risks and fail or are likely to fail a medical weight loss program.7,8 Bariatric operations allow for substantial weight loss, extended weight maintenance, and control or reversal of obesity-related health problems.9,10 Several series have now reported that LRYGBP improves GERD symptoms, but few have included standardized quality of life tools.11,12,13,14,15
The objective of this study was to evaluate the efficacy of LRYGBP as an antireflux procedure on GERD-related symptoms in morbidly obese patients by using a heart-burn-related quality of life score and other standardized outcomes tools.
METHODS
Patients with recalcitrant GERD and a BMI greater than 35 were offered LRYGBP or Nissen fundoplication. Patients who chose LRYGBP were included in this study. An extensive preoperative evaluation, including history and physical examination, the usage of antacid medication and its efficacy, nutritional and psychiatric evaluation, and indicated specialty consultations, was performed before surgery. All the patients had an upper endoscopy or upper gastrointestinal imaging to document and evaluate their GERD severity and upper GI anatomy. Laboratory evaluation included complete blood count, serum chemistries, and thyroid function testing; 24-hour pH monitoring was done in select patients. All patients received preoperative abdominal sonography. If gallstones were detected, laparoscopic cholecystectomy was performed concomitantly.
Patient preparation for surgery consisted of a detailed explanation in written and oral form of the developmental aspect of LRYGBP and its benefits and risks, including short- and long-term complications, side effects, nutritional sequelae, and the possibility of conversion to the open procedure. Informed consent was obtained. Preoperative bowel cleansing and perioperative antibiotics were administered. Prophylaxis against venous thrombosis and pulmonary embolus consisted of perioperative pneumatic compression devices and low-dose subcutaneous heparin.
Data were collected prospectively and verified retrospectively, then entered into a customized computer database. Data sources included office charts, follow-up notes, hospital charts, and patient interview in the outpatient clinic and phone interviews. Parameters included patient demographics, comorbidity, hospital stay, recovery, complications, weight loss, GERD symptoms, and the need for antacids.
Quality of life changes and patient satisfaction were evaluated using the Heart Burn Related Quality of Life (HRQOL) and SF-36 Quality of Life questionnaires. Recovery was defined as the number of days after surgery when patients resumed common activities of daily living, such as driving, shopping, household activities, and employment.
Follow-up weights were obtained from the University of Pittsburgh Surgical Weight Loss Clinic scale with a capacity of 400 kg. On occasion, official weights were obtained from physician office scales or telephone interviews.
Surgical Technique
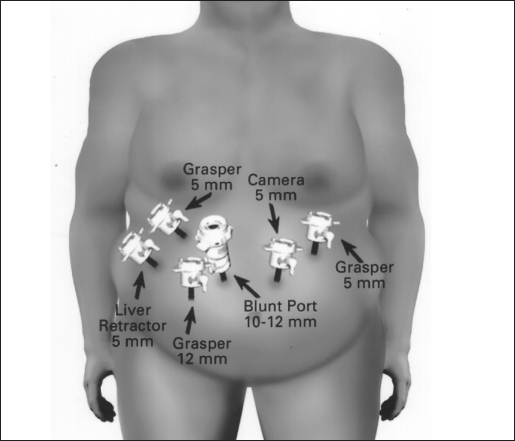
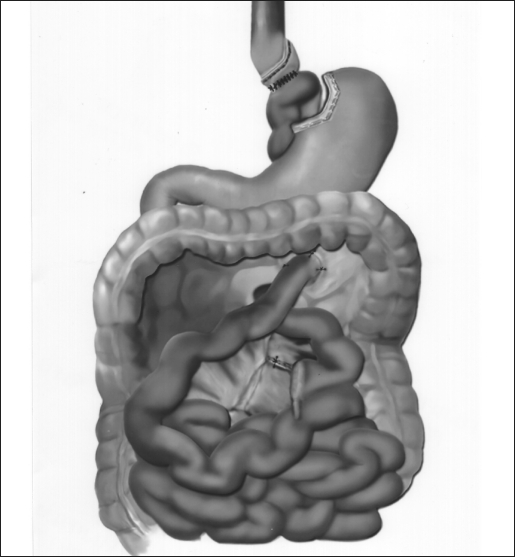
The surgical technique was a modification of the technique described by Wittgrove et al.16 The patient was placed in a supine position with the surgeon on the right and the assistant on the left, and 2 monitors were above the patient's shoulders. After creation of carbon dioxide pneumoperitoneum (15 mm Hg) using the open Hasson technique, cannulas (U.S. Surgical, Norwalk, CT) were placed as shown in Figure 1. The operating table was placed in a steep reverse Trendelenburg position. To expose the esophagus and stomach, a 5-mm liver retractor (Genzyme, Tucker, GA) was placed through the inferior right subcostal port, and the left lateral segment of the liver was elevated. Gastric pouch creation was performed. To localize the esophagogastric junction and size the pouch, a caudate lobe of the liver is a marker for the pouch margins. Then, a window was created in the lesser omentum near the gastric wall at the lesser curvature. The Endo GIA stapler (U.S. Surgical), 60-mm length and 4.8-mm staples, was inserted and applied 3 or 4 times to staple and cut the gastric pouch with 3 rows of staples on each side. A smaller staple size (3.5 mm) was later substituted to reduce staple line bleeds at the transected stomach. The patient was returned to the supine position. The greater omentum and transverse colon were passed to the upper abdomen to expose the ligament of Treitz. To create the Roux limb, the jejunum was transected with an Endo GIA II stapler (U.S. Surgical), 45-mm length and 3.5-mm staples, at approximately 50 cm from the ligament of Treitz, where a comfortable length of mesentery exists. A smaller staple size (2.5 mm) was later substituted to reduce staple line bleeds at the transected bowel. The jejunal mesentery was then divided with 2 applications of the Endo GIA II stapler, using the vascular load (45-mm length, 2.0-mm staples). A 6-cm length of Penrose drain was sewn to the end of the Roux limb using the Endostitch (U.S. Surgical). A retrogastric-retrocolic tunnel for the Roux limb was then created. Using ultrasonic dissection, a window was created in the meso-colon immediately anterior and lateral to the ligament of Treitz to gain access to the lesser peritoneal sac. The Roux limb was then passed in a retrocolic retrogastric fashion to lie next to the gastric pouch (Figure 2). The gastrojejunostomy was then created using the Endo GIA II stapler, and the gastrojejunostomy anastomosis was closed with interrupted 2–0 Surgideck suture material (U.S. Surgical) using the Endostitch. The gastrojejunostomy and enterotomy site were endoscopically inspected and tested for leakage after insufflation and submerging them in irrigation fluid. The patient was returned to the supine position to create the jejunojejunostomy. The Roux limb was then measured 150 to 180 cm distally, and a stapled side-to-side anastomosis was created with the proximal jejunal limb using 1 application of the Endo GIA stapler II (60-mm length, 3.5-mm staples). Later, a 2.5-mm staple cartridge was used. The enterotomy sites were stapled closed, and the mesentery of the jejunojejunostomy was sutured closed (Figure 2).
Figure 1.
The laparoscopic port placements and their use during the procedure.
Figure 2.
The completed gastric bypass: gastric pouch-jejunal anastamosis, the jejunojejunostomy, closure of the mesentery.
Patients began ambulating on the evening of surgery. Pain management consisted of morphine patient-controlled analgesia (PCA) intravenously as needed. An upper gastrointestinal series was performed on the morning of the first postoperative day using Gastrografin followed by barium. A clear liquid diet was begun that day, and the patient was discharged from the hospital after demonstrating tolerance for the diet and return of bowel function, usually on the second postoperative day. Patient follow-up was scheduled for every 2 months, with laboratory evaluation every 6 months, until weight loss stabilized (usually 1 to 1.5 years after surgery), then twice per year. All the patients are routinely treated with a low dose of 150 mg/day Ranitidine hydrochloride, to prevent anastomotic ulcers.
RESULTS
From February 1999 to April 2001, 57 patients with a median BMI of 43 underwent attempted LRYGBP at the thoracic division of the University of Pittsburgh Medical Center. Demographics are listed in Table 1.
Table 1.
Patient Demographics (N=57)
| Sex |
| 51 Females |
| 6 Males |
| Age |
| Mean, 43 |
| Range, 22–67 |
| BMI |
| Median, 43 |
| Range, 35–67 |
Hiatal hernia or esophagitis, or both, were present in 48 patients; Barrett's esophagus was documented in 2. LRYGB was possible in 52 patients; 5 required open conversion, 3 due to multiple adhesions and 2 for poor exposure. The median hospital stay was 3 days (range, 3 to 9). Complications included 1 leak, 1 pulmonary emboli, 2 reoperations for internal roux intestinal limb hernia, and 7 gastrojejunal strictures, which were treated with endoscopic dilatations, average of 1 postoperative dilatation (range, 1 to 3). No deaths occurred. At a mean follow-up of 18 months (range, 3 to 30), all patients report improvement or no symptoms of GERD and a mean weight loss of 40 kg (range, 16 to 70). Previous and current antacid medication usage is summarized in Table 2.
Table 2.
Before and After Surgery Antacid Medication Usage
| Medication | No. of Patients Before Surgery | No. of Patients After Surgery |
|---|---|---|
| High dose proton pump inhibitors | 17 | 0 |
| Proton pump inhibitors 20 mg twice per day | 14 | 3 |
| High dose H2 blockers | 17 | All patients were treated with low dose Ranitidine 150 mg/day |
| Antacids | 9 | 2 |
Quality of life scores (SF-36) were above national norms for physical and mental components (median=55, mode=50). The GERD-health related quality of life median score was <1 (scale, 0 to 45, 0=asymptomatic, 45=worse) (Table 3).
Table 3.
GERD-Health Related Quality of Life Scores (N=40)
| HRQL: Mean, 1.945; Median, 0 |
| HRQL Satisfaction Score: Mean, 1.108; Median, 1.0 |
| SF-36 PCS: Mean, 57.16; Median, 55 |
| SF-36 MCS: Mean, 52.4; Median, 55 |
DISCUSSION
Excessive body weight is a significant independent risk factor for a hiatal hernia and is significantly associated with esophagitis.17 Fisher et al18 demonstrated a correlation between both weight and body mass index with gastroesophageal reflux. Since the early 1990s, the status of laparoscopic antireflux surgery has moved from experimental to routine, with large experiences now reported by many centers.17 Furthermore, the outcome in the majority of patients undergoing surgery is good, with relief of reflux symptoms in about 90% of the patients.20 Laparoscopic surgery for the correction of gastroesophageal reflux has become common throughout the western world,21 partly because of better patient acceptance due to a perception that surgical procedures are now less invasive and also because of an apparently increasing incidence of reflux presenting as a clinical problem.
Our series of patients is the first description and evaluation of the LRYGBP as an antireflux procedure. Previously, it was reported that one of the major morbidities after ring vertical gastroplasty is severe acid reflux22,23; the Roux-en-Y gastric bypass suggests a solution to this complication although it has never been studied from the perspective of treating gastroesophageal reflux disease. All the patients in this study had symptomatic GERD, and they opted to be treated with LRYGBP to address the initial GERD problem and the other comorbidities associated with their morbid obesity. They were all treated with low-dose Ranitidine hydrochloride postoperatively to prevent anastomotic ulcers; however, the dosage used is nontherapeutic for GERD symptoms. The use of antacid medications after the surgery was in less than 10% of the patients, and all of these were on a lower dosage than that prior to the surgery. All our patients were refractory to medical treatment before surgery, and 53% were on a high dosage and a conventional dosage of proton pump inhibitors. All the patients were interviewed, and the heartburn-related symptoms were recorded. According to our results, all were free of GERD symptoms after the operation, and it was not always correlated to their excess body weight lost. Our result may suggest that the antireflux mechanism of this surgery is a combination of acid reflux reduction due to gastric stapling or gastroplasty and the reduction of increased intraabdominal pressure by reduction of excess body weight resulting in lower intraabdominal pressure.
CONCLUSION
LRYGBP was effective for recalcitrant GERD in this patient population. LRYGBP also led to weight loss and improvement in other comorbidities. Thoracic surgeons with laparoscopic expertise should consider LRYGBP for treatment of GERD in the MO.
Footnotes
This paper was presented at the 11th International Congress and Endo Expo, SLS Annual Meeting, New Orleans, Louisiana, USA, September 11-14, 2002.
References:
- 1. Duranceau A, Jamieson GG. Hiatal hernia and gastroesophageal reflux. In: Sabiston DCJ, Lyerly HK. eds. Textbook of Surgery. Philadelphia, PA: WB Saunders; 1997: 767– 783 [Google Scholar]
- 2. Isolauri J, Luostarinen M, Viljakka M, Isolauri E, Keyrilainen O, Karvonen AL. Long-term comparison of antireflux surgery versus conservative therapy for reflux esophagitis. Ann Surg. 1997; 225 ( 3): 295– 299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters JH, DeMeester TR. Indications, benefits and outcome of laparoscopic Nissen fundoplication. Dig Dis. 1996; 14 ( 3): 169– 179 [DOI] [PubMed] [Google Scholar]
- 4. Trus TL, Laycock WS, Branum G, Waring JP, Mauren S, Hunter JG. Intermediate follow-up of laparoscopic antireflux surgery. Am J Surg. 1996; 171 ( 1): 32– 35 [DOI] [PubMed] [Google Scholar]
- 5. Hinder RA, Raiser F, Katada N, McBride PJ, Perdikis G, Lund RJ. Results of Nissen fundoplication. A cost analysis. Surg Endosc. 1995; 9 ( 12): 1328– 1332 [PubMed] [Google Scholar]
- 6. Alverez-Cordero R. Treatment of clinically severe obesity, a public health problem. World J Surg. 1998; 22: 905– 906 [DOI] [PubMed] [Google Scholar]
- 7. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992; 55( suppl): 615S– 619S [DOI] [PubMed] [Google Scholar]
- 8. Brolin RE. Update: NIH consensus conference: gastrointestinal surgery for severe obesity. Nutrition. 1996; 12: 403– 404 [DOI] [PubMed] [Google Scholar]
- 9. MacLean LD, Rhode BM, Sampalis J, Forse RA. Results of the surgical treatment of obesity. Am J Surg. 1993; 165: 155– 162 [DOI] [PubMed] [Google Scholar]
- 10. Mason EE, Renquist KE, Jiang D. Perioperative risks and safety of surgery for severe obesity. Am J Clin Nutr. 1992; 55( suppl); 573S– 576S [DOI] [PubMed] [Google Scholar]
- 11. Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987; 205: 613– 624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deitel M. Laparoscopic bariatric surgery. Surg Endosc. 1997; 11: 965– 966 [DOI] [PubMed] [Google Scholar]
- 13. Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux en-Y: technique and results in 75 patients with 3-30 months follow-up. Obes Surg. 1996; 6: 500– 504 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen NT, Ho HS, Palmer LS, Wolfe BM. Laparoscopic Roux-en-Y gastric bypass for super/super obesity. Obes Surg. 1999; 9: 403– 406 [DOI] [PubMed] [Google Scholar]
- 15. Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996; 171: 74– 79 [DOI] [PubMed] [Google Scholar]
- 16. Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg. 1994; 4: 353– 357 [DOI] [PubMed] [Google Scholar]
- 17. Wilson LJ, Ma W, Hirschowitz BI. Association of obesity with hiatal hernia and esophagitis. Am J Gastroenterol. 1999; 94 ( 10): 2840– 2844 [DOI] [PubMed] [Google Scholar]
- 18. Fisher BL, Pennathur A, Mutnick JL, Little AG. Obesity correlates with gastroesophageal reflux. Dig Dis Sci. 1999; 44 ( 11): 2290– 2294 [DOI] [PubMed] [Google Scholar]
- 19. Peters JH, DeMeester TR. Indications, benefits and outcome of laparoscopic Nissen fundoplication. Dig Dis. 1996; 14 ( 3): 169– 179 [DOI] [PubMed] [Google Scholar]
- 20. Hiebert CA. Surgical management of esophageal reflux and hiatal hernia. Ann Thorac Surg. 1991; 52 ( 1): 159– 160 [DOI] [PubMed] [Google Scholar]
- 21. Van Den Boom G, Go PM, Hameeteman W, Dallemagne B, Ament AJ. Cost effectiveness of medical versus surgical treatment in patients with severe or refractory gastroesophageal reflux disease in the Netherlands. Scan J Gastroenterol. 1996; 31 ( 1): 1– 9 [DOI] [PubMed] [Google Scholar]
- 22. MacLean LD, Rhode BM, Sampalis J, Forse RA. Results of the surgical treatment of obesity. Am J Surg. 1993; 165: 155– 162 [DOI] [PubMed] [Google Scholar]
- 23. Balsiger BM, Murr MM, Sarr MG. Gastroesophageal reflux after intact vertical banded gastroplasty: correction by conversion to Roux-en-Y gastric bypass. J Gastrointest Surg. 2000; 4 ( 3): 276– 281 [DOI] [PubMed] [Google Scholar]