Abstract
Objective:
To evaluate the utility of the holmium laser for partial nephrectomy in a porcine model.
Methods:
Transperitoneal lower pole laparoscopic partial nephrectomy was performed in 5 farm pigs. All animals underwent a left-sided laparoscopic partial nephrectomy and were kept alive for 2 weeks (survival group). Subsequently, a right laparoscopic partial nephrectomy was performed (acute group), and the animals were sacrificed. A 1000-μm (n=6) or 550-μm (n=4) end-fire holmium laser fiber set at 0.2 joules and 60 pulses per second was used to transect the lower pole of the kidney 1 cm below the level of the hilum. The cut parenchymal surface was then sealed with fibrin glue in the survival animals. The operated on kidneys were inspected grossly and evaluated microscopically.
Results:
Laser transection was successfully completed in all cases, and hemostasis proved adequate without any adjunctive measures. No perioperative complications occurred. Estimated blood loss was less than 50 cc for each laparoscopic partial nephrectomy. The acute and survival pigs showed no statistically significant differences in specimen size or weight. Serum creatinine levels were normal in all survival animals. Extravasation was noted on retrograde pyelograms of 2 animals in the survival group.
Conclusions:
The Holmium:YAG laser provides an efficacious modality for transecting the kidney in a porcine model. Clinical trials are necessary to determine its role in laparoscopic partial nephrectomy in humans.
Keywords: Holmium laser, Partial nephrectomy, Laparoscopy, Kidney, Porcine model
INTRODUCTION
The increase in incidentally found small renal tumors has served as an impetus to develop less invasive parenchymal-sparing techniques for tumor resection.1 Recent studies have shown that renal parenchymal-sparing procedures yield comparable outcomes with regard to tumor control compared with outcomes of radical nephrectomy for small tumors.2–5 To reduce the morbidity of partial nephrectomy, several investigators have reported laparoscopic partial nephrectomy (LPN) in select patients with small lesions.6–10 However, adequate hemostasis can be difficult to achieve laparoscopically.
Several hemostatic modalities have been used during LPN with variable success, including argon beam coagulation,11,12 neodymium:YAG laser,12 Harmonic scalpel,13 hand assistance,6 bipolar electrical current,14 unipolar spray electrical current,14 ultrasound scissors,14 microwave tissue coagulation,15 cable-ties,16–21 and radiofrequency ablation.22,23 Based on the success of Holmium: YAG laser prostatectomy, we designed a study to evaluate the feasibility and efficacy of the holmium laser in performing LPN in a porcine model.24
METHODS
In an Animal Care and Use Committee-approved study, laparoscopic transperitoneal lower pole partial nephrectomy was performed using the Holmium:YAG laser in 5 female farm pigs (average weight, 45 to 50 kg). A pilot study in a single animal was used to determine the optimal parameters for the laser. Four separate LPN were performed using the holmium laser at settings of 0.5 joules at 55 pulses/sec, 0.8 joules at 40 pulses/second, or 0.2 joules at 60 pulses/second. The holmium component was used exclusively for the procedures. All settings resulted in successful partial nephrectomy with good hemostasis (estimated blood loss, <50 cc). The setting of 0.2 joules at 60 pulses/second provided an almost continuous delivery of energy, which produced smooth cutting of the kidney and was therefore chosen for the current study.
The Holmium laser was used in all 5 animals to perform laparoscopic transection of the lower pole of the left kidney; 2 weeks after the procedure, a right lower pole laparoscopic partial nephrectomy was performed, and the animals were immediately sacrificed. For the purposes of analysis, the initial left LPN kidneys are referred to as the survival kidneys, and the right LPN kidneys are the acute kidneys.
Surgical Technique
General endotracheal anesthesia was induced using an initial dose of Terazol 4 mg/kg and ketamine 2.2 mg/kg with isoflurane 2% used for maintenance. The pigs were placed in a lateral position. Three ports were placed: a 12-mm trocar just lateral to the rectus muscle at the level of the umbilicus, a 12-mm trocar in the lower lateral quadrant at the midclavicular line, and a 12-mm trocar approximately halfway between the umbilicus and the xiphoid at the midclavicular line. The lower pole of the kidney was mobilized, and the proximal ureter was displaced medially, away from the lower pole of the kidney. The hilum was left undisturbed. A 2-mm port was then inserted under laparoscopic guidance at the level of the lower pole of the kidney to accommodate and stabilize the end-fire Holmium:YAG laser fiber (Trimedyne Corporation, Irvine, CA) (1000 μm [n=6] or 550 μm [n=4]).
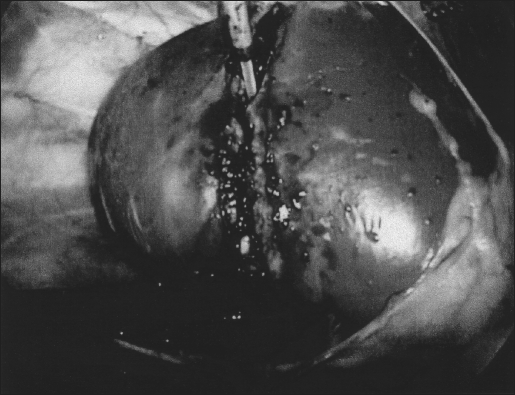
With the laser set at 0.2 joules/pulse and 60 pulses per second, an incision was initiated 1 cm below the level of the hilum with direct laser contact on the parenchyma. First, an arcuate capsulotomy extending from anteromedial to lateral was performed. Then a groove was cut into the cortex of the kidney from medial to lateral (Figure 1), which was deepened until the lower pole was completely excised. Retraction of the cut edges during incision facilitated visualization of the posterior cortex. The laser was defocused as necessary to coagulate small areas of bleeding. By withdrawing the laser from contact with the cut surface, a larger surface area was encompassed by laser energy (defocused), permitting coagulation of any bleeding surface. No additional hemostatic maneuvers were used. Fibrin glue (1 cc) was then applied to the cut edge of the kidney to seal the collecting system. In the animals undergoing left LPN (survival kidney), the specimen was removed intact using an organ entrapment sack via an extension of a trocar site. The animals were immediately euthanized after the right LPN.
Figure 1.
Holmium:YAG laser incising kidney.
Postoperative Protocol
The animals received 1 g of Ancef preoperatively and on postoperative day 1. The animals were observed for a 2-week period. Serum creatinine was obtained on postoperative days 0, 1, 4, and 7. At 14 days, the pigs were anesthetized and a laparoscopic right lower pole partial nephrectomy was performed. The kidneys were then harvested and an in situ left retrograde pyelogram was performed (n=4). Gross inspection and histopathologic evaluation using hematoxylin and eosin staining were performed.
RESULTS
All lower pole partial nephrectomies were successfully completed, and hemostasis was adequate in all cases. The estimated blood loss was less than 50 cc for each partial nephrectomy. Table 1 shows the operative data for the acute and 2-week survival pigs. No statistically significant differences were noted in specimen length (as a percentage of renal length) or weight between the acute and survival groups. The differences in the operative time can be accounted for by the additional time required to apply fibrin glue, remove the specimen, and close the trocar sites in the survival animals. No difference in the cutting or hemostatic ability existed between the 550 and 1000 μm fibers.
Table 1.
Operative Data
| Acute Group Median (Range) | 2-Week Survival Group Median (Range) | |
|---|---|---|
| Total Operative Time (min) | 45 (30–60) | 75 (45–90) |
| Length of Kidney (cm) (Cephalocaudad) | 7.2 (7.0–8.0) | 8.3 (7.2–9.0) |
| Length of Specimen (cm) (Cephalocaudad) | 2.9 (2.2–3.0) | 3.4 (3.1–3.7) |
| Weight of Kidney (g) | 82.9 (73.9–100.1) | 85.8 (76.6–105.6) |
| Weight of Specimen (g) | 17.3 (11.9–21.3) | 19.2 (14.4–23.0) |
No complications occurred in the postoperative period, and all the pigs recovered fully. All serum creatinine levels in the perioperative period were within the normal range.
Left retrograde pyelograms performed on 4 survival animals demonstrated extravasated contrast in 2 animals that was contained by small bowel that adhesed to the cut surface of the kidney. These animals were asymptomatic.
Gross examination of both the acute and 2-week kidneys was performed. The acute amputation sites had a slightly irregular surface contour with minimal amounts of adhesed blood and fibrin. The 2-week kidneys had a similar cut surface without the clotted blood. The renal parenchyma from the acute and 2-week kidneys appeared otherwise normal. Figure 2 shows a representative cut surface of the kidney after an acute and 2-week survival LPN. Microscopic sections from the acute amputation sites showed mild histologic changes including increased cytoplasmic eosinophilia of the renal tubules with blurring of the cytoplasmic borders and elongation of the nuclei. The glomeruli did not show any significant abnormalities. Sections from the 2-week specimens showed a 1.5- to 2.0-mm zone of organized fibrosis with admixed lymphohistiocytic inflammation.
Figure 2.
Resection surface immediately after right lower pole amputation (right). Resection surface of left kidney after 2-week survival (left).
DISCUSSION
With the increase in incidentally detected renal lesions, the indications for partial nephrectomy are expanding. However, the procedure is more technically challenging than radical nephrectomy, both with the open and laparoscopic approaches. In contradistinction to open partial nephrectomy where hilar occlusion limits blood loss, the laparoscopic approach precludes safe hilar occlusion due to the risk of warm renal ischemia. Furthermore, routine suturing of transected vessels during LPN is technically challenging and time-consuming.25 These concerns have prompted a search for techniques that provide safe, reliable hemostasis during LPN.
Winfield and colleagues26 performed the first LPN in 1992 in a patient with a lower-pole stone-bearing caliceal diverticulum. An electrosurgical blade and laparoscopic argon beam coagulator were used to achieve hemostasis, which was further facilitated by closure of Gerota's fascia. Winfield and associates8 subsequently reported on LPN in 6 patients, among whom 2 required open conversion. The argon beam coagulator was also used to achieve hemostasis in this group of patients. Janetschek and colleagues10 used a Neodymium:YAG laser (n=1), argon beam coagulator (n=1), and bipolar coagulation forceps (n=5) along with oxidized cellulose and gelatin resorcinol formaldehyde glue to perform 7 laparoscopic wedge resections for small solid masses (1 cm to 2 cm) without significant complications.
Hoznek and coworkers9 performed retroperitoneal LPN in 13 patients using rotating tip coagulating scissors, bipolar coagulator, Harmonic scalpel, and occasional hilar occlusion to incise the kidney and collagen mesh with gelatin resorcinol formaldehyde glue to achieve hemostasis. Recently, Yoshimura and coworkers15 utilized a microwave tissue coagulator to perform laparoscopic partial nephrectomy in 6 patients with small renal tumors (<25-mm). Also, Wolf et al6 performed 10 LPN using hand assistance in 8 with comparable convalescence to open partial nephrectomy. In these LPN series, blood loss was variable and occasionally substantial (>1400 cc). As can be discerned from the various methods used to achieve hemostasis in these series, an ideal surgical technique has not yet been developed.
Since 1994, the availability of high-powered laser systems has expanded the applications of laser technology from stone fragmentation to tissue ablation and incision.24,27–29 The Holmium:YAG laser has a wavelength that is strongly absorbed by water (2100 nm) and has a 0.5-mm depth of penetration. The ability to cut and ablate with the holmium laser is the result of rapid heating of water in tissue.30 Johnson and colleagues28 found that the area of coagulative necrosis produced with the holmium laser was less than that associated with the Neodymium:YAG laser, although both lasers achieved a similar degree of hemostasis in renal tissue.
In a porcine model, the holmium laser effectively transected renal parenchyma while maintaining hemostasis without the need for hilar dissection or vascular occlusion. Both the 1000-μm and 550-μm laser fibers resulted in successful LPN, but the 1000-μm fiber was easier to use due to greater stiffness that increased manual precision. Several technical problems were addressed as the procedure was developed. First, the 2-mm port was occasionally vented to facilitate removal of smoke that accumulated during laser activation. Second, when the laser was defocused to coagulate bleeding sites, visibility was occasionally obscured by blood spray, requiring that the camera be maintained at a distance to avoid the spray. This is a drawback of using the laser in a “dry” CO2 pneumoperitoneum as opposed to submersed in saline as is the case with “wet” endoscopic cases. Lastly, minimizing traction on the cut surfaces of the kidney reduced mechanical trauma to the kidney and subsequent blood loss. Instead, the cut surfaces were gently separated only during the posterior capsulotomy.
We recognize the limitations of this study. The procedures were performed under optimized conditions, only the lower pole. Resection of medial or posterior tumors may present a greater technical challenge. In addition, the loss of renal function resulting from a unilateral partial nephrectomy is not adequately quantified by serial serum creatinine measurements and would be better assessed with differential renal function from a renogram. Although the holmium laser provided adequate hemostasis, sealing of the collecting system was inconsistent, (2 of 4 animals had extravasation on retrograde pyelogram). The reliability of fibrin glue and other products to consistently seal the peripheral collecting system requires further investigation. Of note, none of the pigs developed clinical symptoms from these minor leaks. In clinical use, a perinephric drain would be placed and perinephric fat would be positioned over the cut surface. Finally, a size differential exists between the pig kidney and the adult human kidney. The holmium laser at lower power settings may not adequately coagulate larger vessels in human kidneys. Our pilot study animal showed that hemostasis was easily attained with higher energy levels (0.5 and 0.8 joules), and these settings may prove more appropriate for human use. In fact, we have successfully performed this technique clinically in 3 patients and found the setting of 0.8 joules was able to achieve adequate hemostasis in adult kidneys (in submission). Further clinical evaluation will be necessary to determine this technique's utility for performing LPN.
CONCLUSION
The Holmium:YAG laser is an effective modality for achieving partial nephrectomy in a porcine model. The procedure is quick, safe, and relatively simple. With further study, this technique may be added to the armamentarium of the laparoscopic renal surgeon.
Acknowledgement:
This investigation was conducted at the Southwestern Center for Minimally Invasive Surgery, which is supported in part by a research grant from United States Surgical Corporation – a division of TYCO Healthcare Group. The Trimedyne Corporation, Irvine, CA provided additional support. We thank the Department of Laser Safety for their assistance with this project.
Contributor Information
Yair Lotan, Department of Urology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
Matthew T. Gettman, Department of Urology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
Guy Lindberg, Department of Pathology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
Cheryl A. Napper, Department of Urology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
John Hoopman, Department of Laser Safety, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
Margaret S. Pearle, Department of Urology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
Jeffrey A. Cadeddu, Department of Urology, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA..
References:
- 1. Smith SJ, Bosniak MA, Megibow AJ, Hulnick DH, Horii SC, Raghavendra BN. Renal cell carcinoma: earlier discovery and increased detection. Radiology. 1989; 170: 699– 703 [DOI] [PubMed] [Google Scholar]
- 2. Licht MR, Novick A C. Nephron sparing surgery for renal cell carcinoma. J Urol. 1993; 149: 1– 7 [DOI] [PubMed] [Google Scholar]
- 3. Thrasher JB, Robertson JE, Paulson DF. Expanding indications for conservative renal surgery in renal cell carcinoma. Urology. 1994; 43: 160– 168 [DOI] [PubMed] [Google Scholar]
- 4. Duque JL, Loughlin KR, O'Leary MP, Kumar S, Richie JP. Partial nephrectomy: alternative treatment for selected patients with renal cell carcinoma. Urology. 1998; 52: 584– 590 [DOI] [PubMed] [Google Scholar]
- 5. Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000; 163: 442– 445 [PubMed] [Google Scholar]
- 6. Wolf JS, Jr, Seifman BD, Montie JE. Nephron sparing surgery for suspected malignancy: open surgery compared to laparoscopy with selective use of hand assistance. J Urol. 2000; 163: 1659– 1664 [DOI] [PubMed] [Google Scholar]
- 7. Gill IS, Delworth MG, Munch LC. Laparoscopic retroperitoneal partial nephrectomy. J Urol. 1994; 152: 1539– 1542 [DOI] [PubMed] [Google Scholar]
- 8. Winfield HN, Donovan JF, Lund GO, et al. Laparoscopic partial nephrectomy: initial experience and comparison to the open surgical approach. J Urol. 1995; 153: 1409– 1414 [DOI] [PubMed] [Google Scholar]
- 9. Hoznek A, Salomon L, Antiphon P, et al. Partial nephrectomy with retroperitoneal laparoscopy. J Urol. 1999; 162: 1922– 1926 [DOI] [PubMed] [Google Scholar]
- 10. Janetschek G, Daffner P, Peschel R, Bartsch G. Laparoscopic nephron sparing surgery for small renal cell carcinoma. J Urol. 1998; 159: 1152– 1155 [PubMed] [Google Scholar]
- 11. Quinlan DM, Naslund MJ, Brendle CB. Application of argon beam coagulation in urological surgery. J Urol. 1992; 147: 410– 412 [DOI] [PubMed] [Google Scholar]
- 12. Malloy TR, Schultz RE, Wein AJ, Carpiniello VL. Renal preservation utilizing neodymium:YAG laser. Urology. 1986; 27: 99– 103 [DOI] [PubMed] [Google Scholar]
- 13. Jackman SV, Cadeddu JA, Chen RN, et al. Utility of the harmonic scalpel for laparoscopic partial nephrectomy. J Endourol. 1998; 12: 441– 444 [DOI] [PubMed] [Google Scholar]
- 14. Barret E, Guillonneau B, Cathelineau X, Validire P, Vallancien G. Laparoscopic partial nephrectomy in the pig: comparison of three hemostasis techniques. J Endourol. 2001; 15: 307– 312 [DOI] [PubMed] [Google Scholar]
- 15. Yoshimura K, Okubo K, Ichioka K, Terada N, Matsuta Y, Arai Y. Laparoscopic partial nephrectomy with a microwave tissue coagulator for small renal tumor. J Urol. 2001; 165: 1893– 1896 [DOI] [PubMed] [Google Scholar]
- 16. McDougall EM, Clayman RV, Chandhoke PS, et al. Laparoscopic partial nephrectomy in the pig model. J Urol. 1993; 149: 1633– 1636 [DOI] [PubMed] [Google Scholar]
- 17. Winfield HN, Donovan JF, Lund GO, et al. Laparoscopic partial nephrectomy: initial experience and comparison to the open surgical approach. J Urol. 1995; 153: 1409– 1414 [DOI] [PubMed] [Google Scholar]
- 18. Beck S, Lifshitz D, Savatta D. The use of the endoloop for laparoscopic partial nephrectomy. J Urol. 2000; 163 ( 5): 347 [Google Scholar]
- 19. Gill IS, Munch LC, Clayman RV, McRoberts JW, Nickless B, Roemer FD. A new renal tourniquet for open and laparoscopic partial nephrectomy. J Urol. 1995; 154: 1113– 1116 [PubMed] [Google Scholar]
- 20. Cadeddu JA, Corwin TS, Traxer O, Collick C, Saboorian HH, Pearle MS. Hemostatic laparoscopic partial nephrectomy: cable-tie compression. Urology. 2001; 57: 562– 566 [DOI] [PubMed] [Google Scholar]
- 21. Cadeddu JA, Corwin TS. Cable tie compression to facilitate laparoscopic partial nephrectomy. J Urol. 2001; 165: 177– 178 [DOI] [PubMed] [Google Scholar]
- 22. Gettman MT, Bishoff JT, Su LM, et al. Hemostatic laparoscopic partial nephrectomy: initial experience with the radiofrequency coagulation-assisted technique. Urology. 2001; 58 ( 1): 8– 11 [DOI] [PubMed] [Google Scholar]
- 23. Corwin TS, Cadeddu JA. Radio frequency coagulation to facilitate laparoscopic partial nephrectomy. J Urol. 2001; 165: 175– 176 [DOI] [PubMed] [Google Scholar]
- 24. Gilling PJ, Mackey M, Cresswell M, Kennett K, Kabalin JN, Fraundorfer MR. Holmium laser versus transurethral resection of the prostate: a randomized prospective trial with 1-year followup. J Urol. 1999; 162: 1640– 1644 [PubMed] [Google Scholar]
- 25. Kozlowski PM, Winfield HN. Laparoscopic partial nephrectomy and wedge resection. J Endourol. 2000; 14: 865– 871 [DOI] [PubMed] [Google Scholar]
- 26. Winfield HN, Donovan JF, Godet AS, Clayman R V. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993; 7: 521– 526 [DOI] [PubMed] [Google Scholar]
- 27. Gilling PJ, Cass CB, Malcolm AR, Fraundorfer MR. Combination holmium and Nd:YAG laser ablation of the prostate: initial clinical experience. J Endourol. 1995; 9: 151– 153 [DOI] [PubMed] [Google Scholar]
- 28. Johnson DE, Cromeens DM, Price RE. Use of the holmium:YAG laser in urology. Lasers Surg Med. 1992; 12: 353– 363 [DOI] [PubMed] [Google Scholar]
- 29. Razvi HA, Chun SS, Denstedt JD, Sales JL. Soft-tissue applications of the holmium:YAG laser in urology. J Endourol. 1995; 9: 387– 390 [DOI] [PubMed] [Google Scholar]
- 30. Erhard MJ, Bagley DH. Urologic applications of the holmium laser: preliminary experience. J Endourol. 1995; 9: 383– 386 [DOI] [PubMed] [Google Scholar]