Abstract
Objective:
To report a series of laparoscopic vesicopsoas hitch procedures performed for the treatment of infiltrative ureteral endometriosis.
Methods:
A retrospective chart review of 6 women with severe endometriosis and ureteral obstruction caused by infiltrative disease of the distal ureter was performed. The patients underwent successful laparoscopic ureteroneocystostomy and vesicopsoas hitch.
Results:
Five of the 6 patients had a history of endometriosis, and their obstructions were diagnosed during prior surgeries. The other patient was diagnosed with severe endometriosis of the rectum, bladder, and ureter at the time of the procedure. She was referred for evaluation of an incidental finding of hydroureter and hydronephrosis. Three patients were treated with gonadotrophin-releasing hormone (GnRH) analog for at least 3 months preoperatively. Five patients had ureteral stents in place prior to the psoas hitch surgery. No intraor postoperative complications occurred. All patients had a normal cystogram performed 10 to 14 days postoperatively prior to Foley catheter removal. Stents were kept in place for 6 to 8 weeks, and an intravenous pyelogram (IVP) was done 2 weeks after removal. All patients had a normal renal ultrasound, computer tomography, or intravenous pyelogram at least 1 year postoperatively.
Conclusion:
Laparoscopic vesicopsoas hitch can be a safe and effective alternative to the laparotomy with the known benefits of laparoscopy.
Keywords: Laparoscopy, Vesicopsoas hitch, Ureteral endometriosis, Ureteroneocystostomy, Ureterovaginal fistula, Complication
INTRODUCTION
Endometriosis uncommonly affects the genitourinary tract. In less than 1%, the disease progresses and causes compression and obstruction of the ureter as a result of infiltrative endometriosis, fibrosis, or both.1,2 Progression may occur without symptoms, causing compromised renal function in as many as 25% to 43% of those with infiltrative ureteral disease.1 General principles of treatment include resection of the diseased ureter and subsequent ureteroureterostomy or ureteroneocystostomy, depending on the location and length of the remaining ureter.2–4 To permit a tension-free anastomosis during a ureteroneocystostomy, sufficient length and mobility of the ureter may be obtained by performing a vesicopsoas hitch procedure or using a Boari flap.5
Operative laparoscopy provides the surgeon with magnified vision and superior exposure for detailed dissection.2 Several studies have shown that laparoscopic techniques may be used successfully to treat endometriosis affecting regions, such as the bowel and genitourinary tract, and to repair ureteral injury.3,6–8 In 1994 we reported our initial case of laparoscopic ureteroneocystostomy.5 We implemented proven techniques of vesicopsoas hitch by laparotomy and modified them for the laparoscopic approach, as previously reported in our first case.3,9 The current series reports laparoscopic ureteroneocystostomy and vesicopsoas hitch in 6 women with distal ureteral disease.
METHODS
Six women were surgically treated for severe infiltrative endometriosis of the distal ureter with laparoscopic resection of the involved segment of the ureter, ureteroneocystostomy, and vesicopsoas hitch. The mean age at the time of surgery was 39 (range, 28 to 50), gravidity 1.66 (range, 0 to 4), and parity 1.66 (range, 0 to 3). Five patients had extensive endometriosis, which had been previously diagnosed in their prior surgeries, and the other was referred for an incidental finding of hydronephrosis detected by a pelvic computer tomography during an evaluation for uterine leiomyomata (Table 1). At the time of this patient's vesicopsoas hitch procedure, she was diagnosed with severe endometriosis of the bladder, rectum, and ureter. Three patients received medical management with at least 3 months of GnRH analog treatment. Ureteral obstruction was established by results of preoperative imaging studies (Table 1). Five patients had ureteral stents in place prior to the ureteroneocystostomy procedure, and the other had stents placed during the procedure.
Table 1.
Results
| Patient | Age | Gravidity/Parity | Preoperative Medications | Preoperative Evaluation (Results) | Location of Obstruction | Postoperative Evaluation | Follow-up Period |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 0/0 | Leuprolide acetate (3 mo) | IVP (hydroureter) | Right (distal to pelvic brim) | Normal cystogram, and renal ultrasound | 33 mo |
| 2 | 48 | 1/1 | None | Pelvic CT (hydronephrosis hydroureter) | Left (distal to pelvic brim) | Normal cystogram, and renal ultrasound | 40 mo |
| 3 | 35 | 0/0 | None | Antegrade pyelogram (prior nephrostomy) | Left (distal portion) 5-6 cm from bladder dome | Normal cystogram, and renal ultrasound | 40 mo |
| 4 | 50 | 2/2 | None | Excretory ureterogram (hydronephrosis) | Left (distal portion near urethro-vesical junction) | Normal cystogram, and IVP | 30 mo |
| Post ureterolysis extravasation noted in surgery 5 days prior—JP drain in | |||||||
| 5 | 38 | 4/3 | Leuprolide acetate (8 mo) | IVP (filling defect ureter) | Right (distal to pelvic brim) | Normal cystogram, and IVP | 28 mo |
| 6 | 37 | 0/0 | Danazol (8 mo) | Pelvic CT (cystic mass, hydronephrosis) | right (proximal to UV junction) | Normal cystogram, and pelvic CT scan | 31 mo |
| Retrograde ureterogram (hydroureter) |
*IVP –intravenous pyelogram; CT–computed tomography scan; UV–utererovesical; Mo–months.
Preoperatively, all patients received a detailed explanation of the operative laparoscopic procedure, including the risks and benefits involved. They also signed a general surgical informed consent for the procedure. As this was part of our routine surgical practice, an approval from the institute's Human Investigation Board was not obtained. Patient confidentiality was maintained at all times.
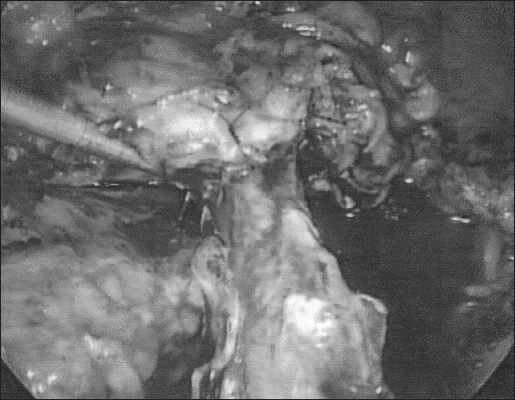
Applying techniques proven effective by laparotomy, the laparoscopic ureteroneocystostomy was performed as follows. Access to the abdominal cavity was obtained in the usual fashion with the multipuncture operative laparoscopy technique.1 Ureterolysis, enterolysis, and adhesiolysis were performed to restore pelvic anatomy1,2 (Figure 1). After consultation with the urologist in attendance, the ureter was transected at the site distal to the healthy segment of the ureter (Figure 2).
Figure 1.
Mobilization of the ureter.
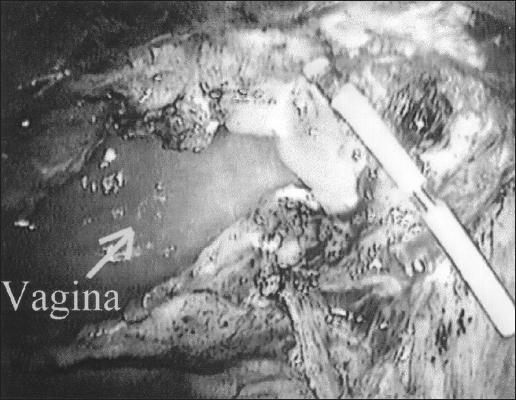
Figure 2.
Ureter transection and passage of the catheter.
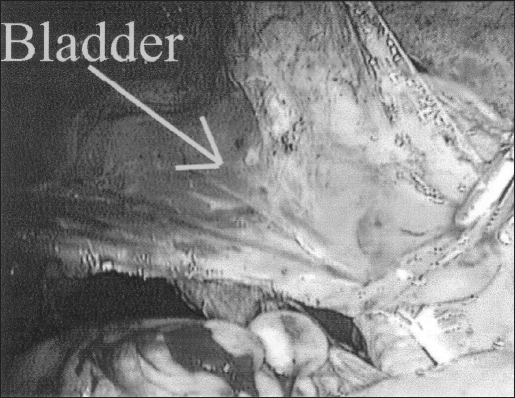
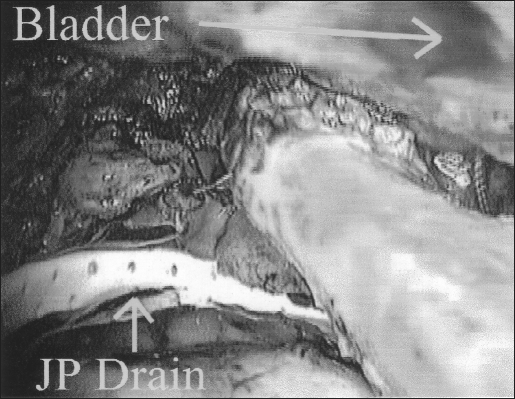
To provide a tension-free ureteroneocystostomy for the remaining length of the ureter, retroperitoneal ureteral mobilization was carried out from above the pelvic brim, and a bladder flap was developed with dissection of vesico, cervico-vaginal fascia. The space of Retzius was entered between the obliterated umbilical ligaments as described for laparoscopic retropubic urethropexy.2,9 Dissection and mobilization proceeded inferiorly to enable the bladder to easily reach the psoas muscle of the affected ureter's side. The peritoneum lateral to the external iliac vessels was incised, and the psoas muscle was exposed. Next, a fishmouth incision was made in the transected ureter with the use of laparoscopic scissors. A 6 F open-ended ureteral catheter was guided cystoscopically into the transected ureter (Figure 2). To decrease the chances of recurrence, the ureter was pulled close to the posterior bladder wall but away from the site of dissection and any potentially residual disease at the ureter bed. With cystoscopic guidance, a neocystostomy site was located and a 1-cm opening was made laparoscopically. In all cases, 4-0 polydioxanone sutures were placed incorporating all layers of tissue to anastomose the ureter to the cystostomy site at the 3, 6, 9, and 12 o’clock positions over the ureteral catheter (Figure 3). The catheter was then changed to a double-J ureteral stent-over guidewire at the conclusion of the procedure. The mobilized bladder was secured to the ipsilateral psoas muscle tendon (Figure 4) with 1 or 2 Polyglycon Vicryl sutures (Ethicon, Sommerville, NJ), carefully avoiding the genitofemoral nerve.2,10 In 3 patients, a Jackson-Pratt (Allegiance Healthcare Corporation, McGraw, Illinois) drain was placed in the pelvis and secured to 1 of the 5-mm cannula sites (Figure 4).
Figure 3.
Ureteroneocyststomy is complete, Jackson-Pratt drain in place.
Figure 4.
Psoas hitch in process.
RESULTS
No intraoperative complications occurred. All patients had an indwelling Foley catheter for approximately 2 weeks postoperatively. Three patients had a Jackson-Pratt drain placed intraperitoneally. No one experienced any major complications postoperatively. One patient experienced mild discomfort due to the ureteral stent, which resolved after its removal. Another suffered from mild right leg pain and right lower quadrant discomfort at the site of her Jackson Pratt drain, which also resolved after its removal. All patients had a normal cystogram performed 2 weeks after surgery prior to Foley catheter removal. Stents were kept in place for 6 to 8 weeks, and an intravenous pyelogram (IVP) was done 2 weeks after removal. All patients had a normal renal ultrasound, CT, or IVP at least 1 year postoperatively. At last follow-up (mean, 33 months; range, 28 to 40 months), all patients were doing well (Table 1).
DISCUSSION
Endometriosis can be a silent disease, especially when affecting the genitourinary tract. As endometriosis advances, the ureter is vulnerable to involvement; therefore, ureters should be evaluated in all patients with pelvic endometriosis.1,11 An IVP, renal ultrasound, and/or cystoscopy may be performed for evaluation of the genitourinary tract.3,9 However, surgical confirmation may be necessary for diagnosis and treatment. Laparoscopy is a superior diagnostic tool. It gives the surgeon a magnified view of the operative field for better microsurgical capabilities.2,9
Superficial endometrial implants of the ureter may be treated by safely excising the lesion using carbon dioxide laser and hydrodissection.1,3,7,9,12 If the implants are deeply embedded in tissue, they can cause extensive fibrosis and subsequent ureteral obstruction. Small ureteral wall lesions can be vaporized, but this may cause pinhole openings. In these cases, ureteral stent placement for a period of 2 to 6 weeks is adequate treatment. Larger ureterostomies can be sutured with subsequent stent placement or at times ureteral resection can be managed by ureteroureterostomy or ureteroneocystostomy. In cases of severe involvement of the distal ureter, transection and diversion are necessary.1,3,11
Early surgical exploration must be performed to minimize loss of kidney function.1 Once ureterolysis is performed, ureteral obstruction may be apparent. Several techniques may be implemented to repair ureteral damage, such as ureteroureterostomy, ureteroneocystostomy with or without psoas hitch, Boari's bladder flap, renal transplantation, or ileal interposition.4 The method of choice depends on several factors, including cause and length of ureteral loss, extent and location of ureteral damage, patient's condition, bladder capacity, and surgeon's experience.4,6,8 Recently, several studies have shown that in experienced hands, surgeons may repair damaged ureters laparoscopically with equal success and a minimal complication rate.2,6,8,11
The ureteroneocystostomy with vesicopsoas hitch procedure was first popularized in 1968 by Harrow13 who believed it was a necessary adjunct to ureteral repair due to gynecologic injuries. The laparotomy method has been proven in recent studies to provide positive long-term results.14 It is reported in urologic studies as a versatile, safe, and ideal method for treatment of lower ureteral disease with minimal complications and a high success rate.5,14 Reported side effects involve placement of psoas muscle sutures with injury to femoral nerve branches, namely the genitofemoral nerve. Because all of these injuries were reported with laparotomies, the authors stated the possibility of the injuries being due to the placement of sidewall retractors.10 A laparoscopic approach would eliminate this theoretical risk especially since visualization is much better with videolaparoscopy.
CONCLUSION
Following the principles of ureteroneocystostomy with vesicopsoas hitch by laparotomy, it is possible to perform this procedure by laparoscopy with a similar outcome to laparotomy and with the known benefits of laparoscopy. Operative laparoscopy, although technical and skill dependent, is associated with better visualization and magnification and is less invasive. In experienced hands it is superior to laparotomy in the management of genitourinary disease. In time, laparoscopy will undoubtedly replace laparotomy in managing many urologic pathologies.
Acknowledgment:
We greatly appreciate the aid of the urology consultants in Atlanta, Georgia and Palo Alto, California.
Contributor Information
Ceana H. Nezhat, Nezhat Medical Center, Atlanta, Georgia, USA..
Shazia Malik, Nezhat Medical Center, Atlanta, Georgia, USA..
Farr Nezhat, Mount Sinai School of Medicine, New York, New York, USA..
Camran Nezhat, Stanford University Medical Center, Palo Alto, California, USA..
References:
- 1. Nezhat CR, Siegler A, Nezhat F, Nezhat CH, Seidman D, Luciano A. eds. Laparoscopic treatment of endometriosis. In: Operative Gynecologic Laparoscopy: Principles and Techniques. 2nd ed. New York, NY: McGraw-Hill; 2000: 169– 209 [Google Scholar]
- 2. Nezhat CR, Nezhat F, Nezhat CH, Nasserbakht F, Rosati M, Seidman D. Urinary tract endometriosis treated by laparoscopy. Fertil Steril. 1996; 66: 920– 924 [PubMed] [Google Scholar]
- 3. Nezhat CH, Nezhat F, Freiha F, Nezhat CR. Laparoscopic vesicopsoas hitch for infiltrative ureteral endometriosis. Fertil Steril. 1999; 71: 376– 379 [DOI] [PubMed] [Google Scholar]
- 4. Nezhat CR, Nezhat F, Nezhat CH. Endometriosis of the intestine and genitourinary tract. In: Szabo Z, Kerstien MD, Lewis JE. eds. Surgical Technology International. Vol 3 San Francisco, CA: Universal Medical Press; 1994: 343– 349 [PubMed] [Google Scholar]
- 5. Nezhat CR, Nezhat F, Admon D, Seidman D, Nezhat CH. Laparoscopic management of genitourinary endometriosis [abstract]. J Am Assoc Gynecol Laparosc. 1994; 1( 4 pt 2): S25. [PubMed] [Google Scholar]
- 6. Tulikangas PK, Gill IS, Falcone T. Laparoscopic repair of ureteral injuries. J Am Assoc Gynecol Laparosc. 2001; 8 ( 2): 259– 262 [DOI] [PubMed] [Google Scholar]
- 7. Nezhat CR, Berger G, Nezhat F, Buttram V, Jr, Nezhat CH. Endometriosis: Advanced Management and Surgical Techniques. New York, NY: Springer-Verlag; 1995: 81– 82, 129–131 [Google Scholar]
- 8. Yokoyama M, Iio S, Iwata H, Takeuchi M. Bilateral ureterovaginal fistula treated by psoas hitch and uretero-appendicocystostomy. J Urol. 1992; 147: 1102– 1104 [DOI] [PubMed] [Google Scholar]
- 9. Sullivan LD, Masterson JS, Wright JE. Vesicopsoas hitch: a versatile procedure. Can J Surg. 1982; 25 ( 1): 26– 29 [PubMed] [Google Scholar]
- 10. Kowalczyk J, Keating M, Ehrlich R. Femoral nerve neuropathy after the psoas hitch procedure. Urology. 1996; 47: 563– 565 [DOI] [PubMed] [Google Scholar]
- 11. Nezhat CR, Nezhat F, Green B. Laparoscopic treatment of obstructed ureter due to endometriosis by resection and ureteroureterostomy: a case report. J Urol. 1992; 148: 865– 868 [DOI] [PubMed] [Google Scholar]
- 12. Gomel V, James C. Intraoperative management of ureteral injury during operative laparoscopy. Fertil Steril. 1991; 55 ( 2): 416– 419 [DOI] [PubMed] [Google Scholar]
- 13. Harrow BR. A neglected maneuver for ureterovesical reimplantation following injury at gynecologic operations. J Urol. 1968; 100 ( 3): 280– 284 [DOI] [PubMed] [Google Scholar]
- 14. Mathews R, Marshall F. Versatility of the adult psoas hitch ureteral reimplantation. J Urol. 1997; 158: 2078– 2082 [DOI] [PubMed] [Google Scholar]