Abstract
Objective:
To determine the prevalence of interstitial cystitis and endometriosis in patients with chronic pelvic pain.
Methods:
A prospective analysis was conducted in 178 women with CPP who presented with bladder base/anterior vaginal wall and/or uterine tenderness, with or without irritative voiding symptoms. The Potassium Sensitivity Test was used to assess bladder epithelial dysfunction. Patients were evaluated with concurrent laparoscopy and cystoscopy with hydrodistention.
Results:
Laparoscopic findings among the 178 patients with chronic pelvic pain supported a diagnosis of endometriosis in 134 (75%) patients, and cystoscopy confirmed a diagnosis of interstitial cystitis in 159 (89%) patients. Both interstitial cystitis and endometriosis were diagnosed in 115 patients (65%). The Potassium Sensitivity Test was positive in 146 (82%) patients, with 140 (96%) of these patients diagnosed with interstitial cystitis and 105 (72%) with endometriosis.
Conclusions:
Results of this prospective study show that interstitial cystitis and endometriosis may frequently coexist in patients with chronic pelvic pain. A positive Potassium Sensitivity Test accurately predicted the presence of interstitial cystitis in 96% of these patients with chronic pelvic pain, as confirmed by cystoscopic hydrodistention. It is necessary to consider the diagnosis of endometriosis and interstitial cystitis concurrently in the evaluation of patients with chronic pelvic pain to avoid unnecessary delay in identifying either condition.
Keywords: Interstitial cystitis, Endometriosis, Chronic pelvic pain
INTRODUCTION
Chronic pelvic pain (CPP) is estimated to affect more than 9 million women in the United States.1 Up to 40% of laparoscopies and 10% to 12% of all hysterectomies are performed for CPP.2,3 In addition to lost productivity and decreased quality of life, the diagnosis and treatment of CPP consumes nearly $3 billion of health care expenditures annually.1
The management of CPP is challenging due to the numerous possible differential diagnoses and contributing factors associated with this condition. Possible differential diagnoses include endometriosis, endosalpingiosis, pelvic adhesions, ovarian remnant syndrome, interstitial cystitis (IC), adenomyosis, and uterine leiomyomatas.4–7 These conditions may present with similar symptoms, and one or more may exist concomitantly in a given patient.8
Endometriosis is one of the more prevalent gynecologic diagnoses among women with CPP, affecting more than half of those patients who receive a diagnosis for their CPP.1,2,5,6,9–11 Symptoms include dyspareunia, cyclic perimenstrual low abdominal pelvic pain, symptom flares after sexual intimacy, and irritative voiding in the case of urinary tract involvement.12 A definitive diagnosis of endometriosis requires visual confirmation of the lesion during laparoscopy, and histologic confirmation of the presence of both ectopic endometrial glands and stroma.12
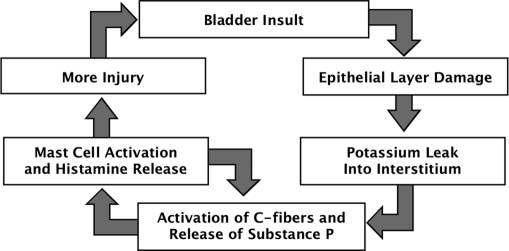
Interstitial cystitis, or pelvic pain of bladder origin, is another disorder that may be associated with CPP.8 The cause of IC is unclear, but it is thought to be multifactorial and progressive, involving bladder epithelial dysfunction, mast cell activation, and bladder sensory nerve upregulation (Figure 1).13–15 Estimates of the prevalence of IC in the United States range from 10 to 510/100 000 cases.16,17 Recent evidence suggests that this condition may, in fact, be much more prevalent than current estimates.14,18
Figure 1.
Proposed cycle of etiology of interstitial cystitis.14 Adapted from Evans RJ. Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol. 2002;4(suppl 1):S16 – S20.
The symptoms of IC include urinary urgency/frequency and/or pelvic pain in the absence of urinary tract infection. Patients may also report dyspareunia and/or cyclic pain in association with the menses.14,19 Some patients may present with only urologic symptoms; conversely, 15% of patients present with chronic pain and no urologic symptoms.14 Patients with early IC may have only 1 or 2 symptoms, whereas at later stages, the full spectrum of symptoms may emerge and bladder capacity may be reduced.20 Recent data suggest that outcomes may be improved when IC is detected and treated at earlier stages.
The Potassium Sensitivity Test (PST) was developed by Parsons et al14,15,21 to indicate abnormal permeability of the bladder epithelium, which is thought to play a central role in the pathophysiology of IC. Use of the PST has been validated in a number of studies,18,19,22,23 and may be used to help confirm the diagnosis of IC, particularly at earlier stages of the disease.
In a previously published retrospective review,9 we diagnosed biopsy-confirmed active endometriosis in 81% of patients with IC who had undergone concurrent laparos-copy and cystoscopy with hydrodistention in evaluation for CPP. Those findings suggested that these 2 conditions often exist concomitantly, and that the diagnosis of one condition should not preclude evaluation for the other. The objective of this study was to incorporate the use of the PST along with cystoscopy/hydrodistention and laparoscopy to prospectively determine the prevalence of IC and endometriosis in patients with CPP.
METHODS
This prospective evaluation included 178 women aged 18 to 60 years who presented from October 2000 to November 2002 at the Midwest Regional Center for Chronic Pelvic Pain and Bladder Control. Subjects were selected for this study based on the presence of abdominal/pelvic, uterine, uterine sacral, and bladder/anterior vaginal wall tenderness during a physical examination. Symptoms of CPP had persisted for more than 6 months and included dyspareunia, dysmenorrhea, and low abdominal pelvic pain with or without irritative voiding symptoms, such as urinary frequency, urgency, nocturia, hesitancy, and sensation of incomplete emptying. Laboratory studies included a urinalysis, urine cytology, and urine and genital cultures. In addition, mycoplasma and urealyticum cultures were performed from the vaginal fornix. Negative culture results, including those for cytology, were required for enrollment.
All patients were tested for bladder potassium sensitivity using the PST as previously described by Parsons et al,21,22 and all patients underwent concurrent laparoscopic, cystoscopic, and bladder hydrodistention evaluations. A diagnosis of endometriosis was confirmed by biopsy and treated by surgical excision. The criteria used for a diagnosis of endometriosis included the pathologist-confirmed presence of ectopic glands and stroma.
Cystoscopy and hydrodistention were performed either before or after the laparoscopic evaluation. Bladder hydrodistention was conducted using sterile water with 80cm to 100cm of passive hydrostatic pressure being applied. The bladder was distended for a minimum of 2 minutes, and then the water was slowly drained. The submucosal petechial hemorrhages, or glomerulations, were observed as the bladder emptied, or during a second filling of the bladder. The diagnosis of IC was based on the National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK) guidelines, including the presence of 10 glomerulations per quadrant in 3 of the 4 quadrants of the bladder cavity and the presence of terminal hematuria.24
RESULTS
Presenting Symptoms
All patients in this study presented with both bladder base and uterine tenderness on physical examination. Overall, 145 (81%) of the 178 study subjects with CPP had irritative urinary symptoms suggestive of overactive bladder.
Results of Cystoscopy with Hydrodistention and Laparoscopy
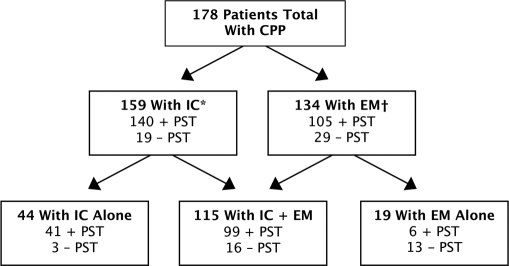
Diagnosis and PST results for patients in this study are shown in Figure 2. Of the 178 patients evaluated, 159 (89%) exhibited cystoscopic findings of IC, 134 (75%) had a diagnosis of endometriosis based on histologic evaluation, and 115 (65%) were diagnosed with both conditions. In addition, 19 (11%) patients had endometriosis with no diagnosis of IC, and 44 (25%) had confirmed IC without endometriosis. The 159 patients with IC met the NIHNIDDK guidelines for IC with the presence of glomerulations and hematuria. The diagnosis of IC was confirmed by experienced urologists who were involved in clinical research for treatment of IC.
Figure 2.
Diagnosis and PST results for patients in this study. CPP=chronic pelvic pain; EM=endometriosis; IC=interstitial cystitis; PST=Potassium Sensitivity Test. *Glomerulations and hematuria according to the National Institutes of Health– National Institute of Diabetes and Digestive and Kidney Diseases guidelines. †Presence of pathologist-confirmed presence of ec-topic gland stroma.
Results of Potassium Sensitivity Testing–Relationship With Cystoscopic Results
Among the total study population of 178 patients, a positive PST was found in 146 (82%) patients. Of these 146 patients, 114 (78%) had irritative voiding symptoms. In addition, cystoscopic evidence of IC was found in 140 (96%) patients and, based on laparoscopic findings, endometriosis was demonstrated in 105 (72%).
In the original subgroup of 159 patients with cystoscopic evidence of IC, 140 (88%) showed increased potassium sensitivity based on the PST, and 129 (81%) had urologic symptoms of urinary urgency and frequency. Cystoscopic evidence of IC was found in 19 (12%) patients who had a negative PST, and 15 of those patients presented with irritative voiding symptoms.
DISCUSSION
In this study, we have shown that endometriosis and IC often exist concomitantly in patients with CPP who present with uterine and bladder tenderness. Among the 134 patients with endometriosis, 86% were also diagnosed with IC. Conversely, among the 159 patients with IC, 72% were also found to have endometriosis. We have therefore prospectively confirmed our earlier published results that IC and endometriosis have a high rate of overlap in this group of patients. As the patients in this study were selected for both uterine and bladder tenderness, the rate of overlap between gynecologic and urologic diagnoses is likely to be higher than in the general population of patients with CPP. However, these results emphasize the need for a careful physical examination and symptom history in patients with CPP, and the need to perform concurrent cystoscopy with hydrodistention and laparos-copy. If cystoscopy had been performed only in patients with irritative voiding symptoms/overactive bladder, a diagnosis of IC would have been missed in approximately 20% of patients.
Other studies have also found that IC is frequently diagnosed in patients with CPP, and that it overlaps to a high degree with other diagnoses. In one study25 of 45 patients from general gynecologic practices who were scheduled to undergo laparoscopy for CPP, 17 (38%) were found to have IC based on the presence of irritative voiding symptoms and positive cystoscopic findings. In that study, 7 (41%) women diagnosed with IC also had endometriosis. Endometriosis was also diagnosed in 14 (50%) women who did not have IC. Notably, 30 (67%) of the women in that study had clinical symptoms of urgency and frequency or nocturia, and 10 women who were IC-negative had at least 10 glomerulations per quadrant noted in only 2 quadrants of the bladder. These results suggest that some women may have had early IC that did not meet the stringent NIDDK criteria for a diagnosis of IC.25
A questionnaire-based study evaluated the prevalence of concomitant disease in individuals with diagnosed IC.26 In this study in which patients with IC were not selected for CPP or gynecologic symptoms, the prevalence of endometriosis was approximately 17%. By comparison, the prevalence of endometriosis in the general population is estimated to be 1% to 7%.12 In this survey, patients with IC were also found to be substantially more likely than the general population to have various autoimmune disorders, such as inflammatory bowel disease (100-fold greater risk) and systemic lupus erythematosus (30- to 50-fold greater risk), although these conditions were still relatively rare compared with other concomitant illnesses. The cause of both IC and endometriosis is thought to include an immune component, suggesting a possible pathophysiologic basis for the increased risk of one condition with the other.26
In this study, we have shown that the PST is reasonably good at detecting the presence of IC: 96% (140) of patients with a positive PST had cystoscopic findings compatible with IC. Only 3.4% (6) of patients with a positive PST were cystoscopically negative. Previous studies have also reported that the rate of false positives is very low with the PST.22,23,27–29 These results indicate that a positive PST may be sufficient to indicate a diagnosis of IC.22 However, in our study, 12% (19) of women with cystoscopically confirmed IC had a negative PST, suggesting that, in the case of a negative PST, cystoscopy should still be performed.
Simple conservative methods are available for treating IC, including diet, pelvic floor physical therapy, and medications like hydroxyzine and pentosan polysulfate sodium (Elmiron®, Ortho-McNeil Pharmaceutical, Inc, Raritan, NJ). Recent published articles have indicated the efficacy of oral contraceptives in treating IC and CPP.30,31 Many thought-leaders believe that the treatment of women with CPP has often been ineffective because the underlying cause is actually urologic rather than gynecologic.32 Therefore, it is reasonable to conclude that ineffective management of CPP and treatment failures may be the result of missed diagnoses of IC.
CONCLUSION
This study demonstrates that patients with CPP may have IC, endometriosis, or both conditions concomitantly. It is therefore imperative to evaluate the patient for both conditions, even when the diagnosis of one has been confirmed. When possible, cystoscopy with hydrodistention should be performed concurrently with laparoscopy to eliminate the need for a second procedure with the patient under general anesthesia and to facilitate the rapid detection and treatment of IC. A positive PST may be considered sufficient to diagnose IC and to begin treatment. A negative PST is not sufficient to rule out the presence of IC and should be followed up with cystos-copy. In the evaluation of the female with CPP, consideration should be given to the bladder as a source of pain early in the diagnostic workup.
Footnotes
Disclosure: Dr. Chung is a member of the speakers bureau for Ortho-McNeil. He has received funding from AstraZeneca, Ortho-McNeil, Pfizer, and Yamanuchi. He is a consultant and a member of the speakers bureau for Bard, Lilly, Medtronic, Ortho-McNeil, and Pfizer
References:
- 1. Mathias SD, Kuppermann M, Liberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321–327 [DOI] [PubMed] [Google Scholar]
- 2. Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv. 1993;48:357–387 [DOI] [PubMed] [Google Scholar]
- 3. Reiter RC. A profile of women with chronic pelvic pain. Clin Obstet Gynecol. 1990;33:130–136 [PubMed] [Google Scholar]
- 4. Howard FM. Introduction. In: Howard FM. ed. Pelvic Pain Diagnosis and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:3–6 [Google Scholar]
- 5. Carter JE. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J Am Assoc Gynecol Laparosc. 1994;2:43–47 [DOI] [PubMed] [Google Scholar]
- 6. Ripps BA, Martin DC. Focal pelvic tenderness, pelvic pain and dysmenorrhea in endometriosis. J Reprod Med. 1991;36:470–472 [PubMed] [Google Scholar]
- 7. Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298 [DOI] [PubMed] [Google Scholar]
- 8. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594–611 [DOI] [PubMed] [Google Scholar]
- 9. Chung MK, Chung RP, Gordon D, Jennings C. The evil twins of chronic pelvic pain syndrome: endometriosis and interstitial cystitis. JSLS. 2002;6:311–314 [PMC free article] [PubMed] [Google Scholar]
- 10. Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759–765 [DOI] [PubMed] [Google Scholar]
- 11. Mettler L, Schollmeyer T, Lehmann-Willenbrock E, et al. Accuracy of laparoscopic diagnosis of endometriosis. JSLS. 2003; 7:15–18 [PMC free article] [PubMed] [Google Scholar]
- 12. Howard FM, El-Minawi AM, Li R-Z. Endometriosis and endosalpingiosis. In: Howard FM, Perry CP, Carter JE, et al. eds. Pelvic Pain Diagnosis & Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2000:125–149 [Google Scholar]
- 13. Evans RJ. Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol. 2002;4(suppl 1):S16–S20 [PMC free article] [PubMed] [Google Scholar]
- 14. Parsons CL, Stanford EJ, Kahn BS, Sand PK. Tools for diagnosis and treatment. Female Patient. May 2002;(suppl):12–17 [Google Scholar]
- 15. Erickson DR. Interstitial cystitis: update on etiologies and therapeutic options. J Womens Health Gend Based Med. 1999;8:745–758 [DOI] [PubMed] [Google Scholar]
- 16. Waxman JA, Sulak PJ, Kuehl TJ. Cystoscopic findings consistent with interstitial cystitis in normal women undergoing tubal ligation. J Urol. 1998;160:1663–1667 [PubMed] [Google Scholar]
- 17. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49(suppl 5A):2–9 [DOI] [PubMed] [Google Scholar]
- 18. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60:573–578 [DOI] [PubMed] [Google Scholar]
- 19. Parsons CL, Zupkas P, Parsons JK. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology. 2001;57:428–433 [DOI] [PubMed] [Google Scholar]
- 20. Ramahi AJ, Richardson DA. A practical approach to the painful bladder syndrome. J Reprod Med. 1990;35:805–809 [PubMed] [Google Scholar]
- 21. Parsons CL. Evidence-based strategies for recognizing and managing IC. Contemp Urol. 2003;15:22–35 [Google Scholar]
- 22. Parsons CL, Bullen M, Kahn BS, Stanford EJ, Willems JJ. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127–132 [DOI] [PubMed] [Google Scholar]
- 23. Parsons CL, Greenberger M, Gabal L, Bidair M, Barme G. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–1867 [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Diabetes and Digestive and Kidney Diseases, National Kidney and Urologic Diseases Information Clearinghouse Interstitial cystitis. National Institutes of Health; Available at: www.niddk.nih.gov/health/urolog/pubs/cystitis/cystitis.htm Accessed: November 3, 2003 [Google Scholar]
- 25. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002; 100:337–341 [DOI] [PubMed] [Google Scholar]
- 26. Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Inter-stitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49(suppl 5A):52–57 [DOI] [PubMed] [Google Scholar]
- 27. Parsons CL, Stein PC, Bidair M, Lebow D. Abnormal sensitivity to intravesical potassium in interstitial cystitis and radiation cystitis. Neurourol Urodyn. 1994;13:515–520 [DOI] [PubMed] [Google Scholar]
- 28. Parsons CL. Potassium sensitivity test. Tech Urol. 1996;2:171–173 [PubMed] [Google Scholar]
- 29. Parsons CL, Benson G, Childs SJ, Hanno P, Sant GR, Webster G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol. 1993;150:845–848 [DOI] [PubMed] [Google Scholar]
- 30. Lentz GM, Bavendam T, Stenchever MA, Miller JL, Small-dridge J. Hormonal manipulation in women with chronic, cyclic irritable bladder symptoms and pelvic pain. Am J Obstet Gynecol. 2002;186:1268–1273 [DOI] [PubMed] [Google Scholar]
- 31. Westney OL, Amundsen CL, McGuire EJ. Bladder endometriosis: conservative management. J Urol. 2000;163:1814–1817 [DOI] [PubMed] [Google Scholar]
- 32. Mattox JH, Parsons CL, Sand PK. Chronic pelvic pain of bladder origin. Female Patient. 2002;(suppl):3 [Google Scholar]