Abstract
Objective:
The scarred or obliterated anterior cul-de-sac may pose a challenge to hysterectomy by any route. Conventional laparoscopic hysterectomy is fraught with technical limitations that limit the ability to compensate for the altered anatomy. This study will evaluate the feasibility of applying robot-assisted laparoscopy to managing these patients.
Methods:
Six patients with suspected pelvic adhesive disease involving the anterior cul-de-sac underwent robot-assisted laparoscopic hysterectomy for benign indications. Data were collected and analyzed as a retrospective case series analysis.
Results:
We attempted 6 robot-assisted laparoscopic hysterectomies with no conversions to laparotomy. The mean uterine weight was 121.7g (range, 70 to 166.3). Mean operating time was 254 minutes (range, 170 to 368). The average estimated blood loss was 87.5 mL. One patient developed a delayed vaginal cuff hematoma. The average length of hospital stay was 1.3 days.
Conclusion:
Robot-assisted laparoscopic hysterectomy is a feasible technique in patients with a scarred or obliterated anterior cul-de-sac and may provide a tool to overcome the surgical limitations seen with conventional laparoscopy.
Keywords: Robot-assisted laparoscopy, Laparoscopic hysterectomy, Pelvic adhesions, Surgical technique
INTRODUCTION
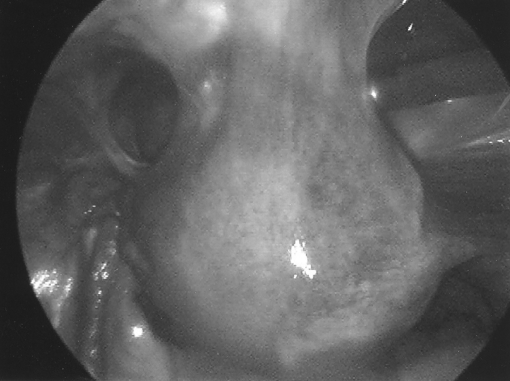
Approximately 600 000 hysterectomies are performed annually in the United States with the majority due to benign conditions.1–3 Before the introduction of laparoscopic-assisted vaginal hysterectomy in the late 1980s, hysterectomies were approached by either a vaginal or abdominal route.4 Since the 1990s, a definite trend toward laparoscopic hysterectomy has been seen. Despite the increasing acceptance of laparoscopy, hysterectomy via laparotomy remains the most common route. This is particularly true in cases of advanced pathology, such as pelvic adhesions of which the scarred or obliterated anterior cul-desac is one example (Figure 1). This finding is known to pose a challenge by any route. The ability to complete a hysterectomy in a minimally invasive fashion is affected not only by the surgical anatomy field but also by the surgeon's skill level and the technical limitations of conventional laparoscopic instruments.5 We view the use of robot-assisted technology as a means to overcome these surgical limitations by providing surgeons with improved dexterity and precision coupled with advanced imaging that allows for the completion of complex minimally invasive procedures.
Figure 1.
Laparoscopic view of partially obliterated anterior cul-de-sac with uterine to anterior abdominal wall adhesions.
The da Vinci Robotic Surgical System (Intuitive Surgical) is a laparoscopic-assist device that is designed to address many of the current limitations of conventional laparoscopy. It is comprised of 3 components. The first component is the surgeon console that is located remotely from the patient bedside. The surgeon seated at this console is able to control robot-assisted instruments within the patient with the aide of a stereoscopic viewer, hand manipulators and foot pedals. The second component of the da Vinci system is the InSite vision system that provides 3-dimensional imaging through a 12-mm, dual optical endoscope. The third component of the da Vinci system is the patient-side cart with robotic arms and Endowrist instruments. Currently, this system is available with either 3 or 4 robotic arms. One of the arms holds the endoscope while the other 2 to 3 arms hold the various 8-mm Endowrist instruments. These Endowrist instruments are unique in that they possess a wrist-like mechanism that allows 7 degrees of movement, thereby replicating the full range of motion of the surgeon's hand and in turn eliminating the fulcrum effect seen with conventional laparoscopy. A series of Endowrist instruments, such as DeBakey forceps and needle drivers, can be interchanged on either of the lateral robotic arms.
Recently, the application of robotic technology to facilitate minimally invasive surgery has increased. In numerous studies, it has been shown to be a safe and effective alternative to conventional laparoscopic surgery. In the gynecology literature are reports of robot-assisted laparos-copy for ovarian transposition, tubal reanastomosis, and hysterectomy.6–10 Although a published report of robot-assisted laparoscopic hysterectomy already exists, all cases in that series were type IIB according to the American Association of Gynecologic Laparoscopists' (AAGL) classification system for laparoscopic hysterectomy.11 This means that the posterior culdotomy and ligation of the cardinal and uterosacral ligament complexes were performed vaginally to complete the hysterectomy. Our hysterectomy series comprises either AAGL type IVE (totally laparoscopic removal of the uterus and cervix including vaginal cuff closure) or LSH III (totally laparoscopic supracervical hysterectomy with removal of the uterine corpus and division of the uterine arteries). We report on the application of robot-assisted laparoscopic hysterectomy to patients with a scarred or obliterated anterior cul-de-sac.
METHODS
A retrospective chart review for data abstraction was performed after obtaining approval from our Institutional Review Board (IRB #2003–0763). Six patients with suspected uterine to anterior abdominal wall adhesive disease who required hysterectomy for benign indications were recommended for a hysterectomy by robot-assisted laparoscopy. The suspected alteration in the anatomical operating field was thought to be a relative contraindication to conventional laparoscopy. A scarred or obliterated anterior cul-de-sac was confirmed by laparoscopy intraoperatively. In all patients, the suspected predisposing factor was previous cesarean delivery. The authors at the University of Michigan Medical Center performed all procedures between January 2002 and January 2003.
Operative Technique
All patients were placed in a low dorsal lithotomy position with arms padded and tucked at their sides after general endotracheal anesthesia was administered. The bladder was drained with a Foley catheter, and the stomach was evacuated with a nasogastric tube. A RUMI uterine manipulator was placed in conjunction with a Koh colpotomy ring and vaginal pneumo-occluder balloon. Pneumoperitoneum was obtained with a Veress needle followed by placement of 4 trocars. A 12-mm port was placed either at or above the umbilicus depending on the size of the uterus. This port accommodated the dual optical endoscope. Two 8-mm ports that mount directly to the operating arms on the patient-side cart were placed in the left and right lower quadrants, respectively. A fourth port served as an accessory port and was placed between the camera port and the right lower quadrant port. This was typically a 12-mm port to facilitate introduction of suture as well as instruments for retraction, suction/irrigation, and specimen removal.
Once all 4 ports were in place, the patient was placed in a steep Trendelenburg position, and the patient-side cart with robotic arms was brought between the patient's legs and docked. Each port was attached to the assigned robotic arm with the exception of the accessory port. The bedside surgeon, at the right side of the patient, was responsible for Endowrist instrument exchanges and any accessory port activity such as introduction of suture.
A survey of the operative field was performed and confirmation of a scarred or obliterated anterior cul-de-sac was made in all 6 patients. As a result of pelvic adhesive disease, attention was turned towards normalization of anatomy before initiation of the hysterectomy. A DeBakey forceps and round-tip scissors, both Endowrist instruments, were attached to the left and right operating arms respectively and used to perform the adhesiolysis. In cases where the anterior cul-de-sac was completely obliterated by uterine to anterior abdominal wall adhesions or the vesico-uterine reflection was significantly scarred, the bladder was filled with 180mL to 240mL of methylene blue stained saline to facilitate dissection. When needed, an Endowrist instrument was exchanged for either a needle driver or monopolar cautery hook. Upon completion of the adhesiolysis, the approach to hysterectomy was carried out in a fashion analogous to the open surgical technique. All of the procedures were consistent with either AAGL type IVE or LSH III laparoscopic hysterectomies.11 All vascular pedicles including the infundibulopelvic ligament and the uterine artery pedicles were skeletonized and subsequently suture ligated with either 0-Vicryl on CT-2 needles or free ties of 0-Vicryl before transection. Countertraction was provided by the bedside surgeon assistant through the accessory port with an atraumatic grasper. Adequate hemostasis was obtained with the combination of suture ligation and electrocautery. In cases where a total laparoscopic hysterectomy was intended, the monopolar cautery hook was utilized to divide the cardinal and uterosacral ligament complex bilaterally. Completion of both the anterior and posterior culdotomy was facilitated by the Koh colpotomy ring while upward uterine traction was provided by the bedside assistant. Pneumoperitoneum was maintained by inflation of the vaginal pneumo-occluder balloon. Once the uterus and cervix were completely detached, the specimen with or without adnexae was delivered into the vagina. The uterine fundus was used to maintain pneumoperitoneum during the closure of the vaginal cuff, which was closed with interrupted sutures of 0-Vicryl on CT-2 needles. All knots were tied intracorporeally. Once the vaginal cuff was closed, the specimen was removed from the vagina. A low-pressure check was performed to ensure hemostasis and the robot-assist device was undocked.
In cases where laparoscopic subtotal hysterectomy was intended, the monopolar cautery hook was used to amputate the uterine corpus below the internal os followed by extraction of the specimen through the accessory port with a tissue morcellator. The cervical stump was closed with interrupted sutures of 0-Vicryl on CT-2 needles.
RESULTS
We attempted 6 cases with zero conversions to laparotomy. Five patients underwent a total laparoscopic hysterectomy (AAGL type IVE), and one patient underwent a laparoscopic subtotal hysterectomy (AAGL type LSH III) in accordance with her wishes. Patients underwent hysterectomy for several benign indications (Table 1). Three patients also underwent a bilateral salpingo-oophorectomy.
Table 1.
Preoperative and Intraoperative Data
| Patient | Indication | Prior Pelvic Surgery | Robotic Procedure* |
|---|---|---|---|
| 1 | Chronic pelvic pain, endometriosis | Cesarean section × 3, tubal ligation, | TLH |
| diagnostic laparoscopy, | |||
| appendectomy | |||
| 2 | Abnormal uterine bleeding | Cesarean section × 5 | TLH, BSO |
| 3 | Abnormal uterine bleeding | Cesarean section × 2, laparoscopic | TLH, BSO |
| tubal ligation | |||
| 4 | Abnormal uterine bleeding | Cesarean section × 4 | LSH |
| 5 | Symptomatic leiomyomata | Cesarean section × 2, laparoscopic | TLH |
| tubal ligation | |||
| 6 | Abnormal uterine bleeding, dysmenorrhea | Cesarean section × 1 | TLH, BSO |
BSO = bilateral salpingo-oophorectomy; TLH = total laparoscopic hysterectomy, AAGL type IV-E; LSH = laparoscopic subtotal hysterctomy, AAGL LSH III.
The mean age was 39.8 years (range, 28 to 44). The mean body mass index was 26.0 kg/m2 (range, 20.3 to 35.0). Estimated blood loss (EBL) was calculated by noting the difference between the volumes of aspirated and irrigated fluids. The mean estimated blood loss was 87.5mL (range, 50 to 150). No blood transfusions were administered in our series. The average uterine weight was 121.7g (range, 70 to 166.3). The mean operating time was 254 minutes (range, 170 to 368). The average hospital stay for all patients in our series was 1.3 days (range, 1 to 2).
The only complication in this series was one patient with a delayed vaginal cuff hematoma that was managed conservatively. No cystotomies were performed.
DISCUSSION
Pelvic adhesions occur following the vast majority of surgical procedures. In fact, it has been recognized that pelvic adhesions occur in 55% to 95% of women following laparotomy.12 This is true despite variables like meticulous surgical technique, laparoscopic approach, and the use of medical and surgical adjuvants. Although the cause of pelvic adhesions is not well known, several risk factors do exists. These include infection, tissue hypoxia or ischemia, tissue desiccation, trauma caused by rough handling of tissue during surgery, foreign body reaction, previous adhesiolysis, and the presence of intraperitoneal blood.13 Many surgeons advocate closure of the peritoneum at the time of laparotomy as a way to avoid or minimize adhesion formation. As a result of this, closure or nonclosure of the peritoneum has been an area of debate in the literature. This study is not designed to address those issues.
The purpose of this study was to review our experience with applying robotic technology to laparoscopic hysterectomies suspected of having altered anatomy secondary to pelvic adhesive disease. Although the cases in our series could potentially have been completed by conventional laparoscopy, we believe that completion of these cases was greatly facilitated by the robotic system. Prior cesarean delivery (range, 1 to 5) was thought to be the key predisposing factor for the adhesions that were present in all 6 of these patients (Table 1). Preoperatively, all of the patients in this study were suspected of having pelvic adhesions involving the anterior cul-de-sac based on physical examination findings.
Pathology involving the anterior aspect of the uterus has been known to pose difficulties to conventional laparos-copy. This is reported in laparoscopic myomectomies where anteriorly located leiomyomata are an important predictor of conversion to laparotomy and are poorly accessible to trocars, particularly when suturing.14 The combination of 3-dimensional imaging and the mechanical-wrist instruments of the da Vinci Robotic Surgical System provided a means by which the altered anatomical operating field of the anterior cul-de-sac could be navigated. Lysis of adhesions as well as development of the vesicouterine reflection was readily accomplished without injury to the bladder or excessive blood loss.
One limitation of the system in its current form is the absence of tactile feedback (haptics) to the surgeon operating the Endowrist instruments remotely at the surgeon's console. Direct tactile sensation often is necessary during cases of difficult adhesiolysis. Despite this, the da Vinci Surgical System shows promise in this aspect of surgery. As technology evolves, this limitation will need to be addressed.
Although operative times were much longer than those in most published studies of conventional laparoscopic hysterectomy, blood loss, complication rates, and lengths of hospital stay were comparable if not better than those reported in other studies.15–17 We attribute the vast majority of the increased operating time to the absence of tactile feedback and believe that with increasing experience, our operative time will decrease.
CONCLUSION
Although no absolute contraindications for laparoscopic hysterectomy exist, a surgeon's experience and the pathology encountered remain the limiting factors for performing laparoscopic hysterectomy.5 Pathology in the form of pelvic adhesions can present a significant challenge to the surgeon attempting a hysterectomy with conventional laparoscopy. This is the first series to report the technique and outcome for robot-assisted laparoscopic hysterectomy in patients with scarred or obliterated anterior culs-de-sac. We believe that with the aide of robot-assisted technology, such as the da Vinci Robotic Surgical System, the limitations of conventional laparoscopy can be overcome. Our preliminary experience indicates that complex pathology can be managed in a minimally invasive fashion with robot-assisted laparoscopy. Despite these advancements in technology and surgical approach, issues like the absence of tactile feedback and cost will need to be addressed.
References:
- 1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States, 1990 –1997. Obstet Gynecol. 2002;99:229–234 [DOI] [PubMed] [Google Scholar]
- 2. Wilcox LS, Koonin LM, Pokras R, et al. Hysterectomy in the United States, 1988 –1990. Obstet Gynecol. 1994;83:549–555 [DOI] [PubMed] [Google Scholar]
- 3. Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance –United States, 1980 –1993. MMWR CDC Surveill Summ. 1997;46:1–15 [PubMed] [Google Scholar]
- 4. Reich H, Decaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213–216 [Google Scholar]
- 5. Wattiez A, Cohen SB, Selvaggi L. Laparoscopic hysterectomy. Cur Opin Obstet Gynecol. 2002;14:417–422 [DOI] [PubMed] [Google Scholar]
- 6. Molpus KL, Wedergren JS, Carlson MA. Robotically assisted endoscopic ovarian transposition. JSLS. 2003;7:59–62 [PMC free article] [PubMed] [Google Scholar]
- 7. Falcone T, Goldberg J, Garcia-Ruiz A, et al. Full robotic assistance for laparoscopic tubal anastomosis: a case report. J Laparoendosc Adv Surg Tech. 1999;9:107–113 [DOI] [PubMed] [Google Scholar]
- 8. Falcone T, Goldberg JM, Margossian H, Stevens L. Robotically assisted laparoscopic microsurgical anastomosis: a human pilot study. Fertil Steril. 2000;73:1040–1042 [DOI] [PubMed] [Google Scholar]
- 9. Degueldre M, Vandromme J, Huong PT, Cadiere GB. Robotically-assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000;74:1020–1022 [DOI] [PubMed] [Google Scholar]
- 10. Diaz-Arrastia C, Jurnalov C, Gomez G, Townsend C., Jr Laparoscopic hysterectomy using a computer-enhanced surgical robot. Surg Endosc. 2002;16(9):1271–1273 [DOI] [PubMed] [Google Scholar]
- 11. Olive DL, Parker WH, Cooper JM, Levine RL. The AAGL classification system for laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 2000;7:9–15 [DOI] [PubMed] [Google Scholar]
- 12. Diamond MP. Surgical aspects of infertility. In: Sciarra JJ, ed. Gynecology and Obstetrics. Philadelphia, PA: Harper & Row; 1995;1–26 [Google Scholar]
- 13. El-Mowafi DM, Diamond MP. Are pelvic adhesions preventable? Surg Tech Int. 2004;XI:222–235 [PubMed] [Google Scholar]
- 14. Dubuisson JB, Fauconnier A, Fourchotte V, et al. Laparoscopic myomectomy: predicting the risk of conversion to an open procedure. Hum Reprod. 2001;16:1726–1731 [DOI] [PubMed] [Google Scholar]
- 15. Makinen J, Johansson J, Tomas C, et al. Morbidity of 10,110 hysterectomies by type of approach. Hum Reprod. 2001;16:1473–1478 [DOI] [PubMed] [Google Scholar]
- 16. Garry R, Fountain J, Mason S, Hawe J, et al. The eVALuate study: two parallel randomized trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meltomaa SS, Makinen JI, Taalikka MO, Helenius HY. One year cohort of abdominal, vaginal, and laparoscopic hysterectomies: complications and subjective outcomes. J Am Coll Surg. 1999;189:389–396 [DOI] [PubMed] [Google Scholar]