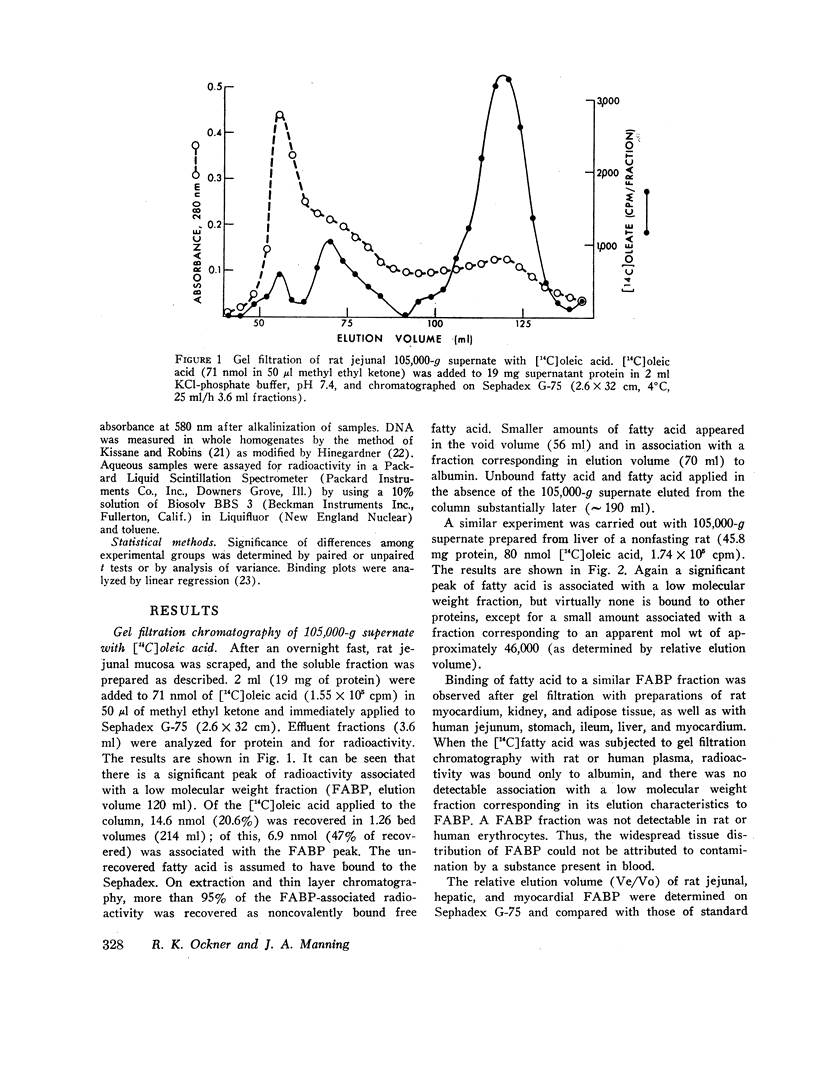
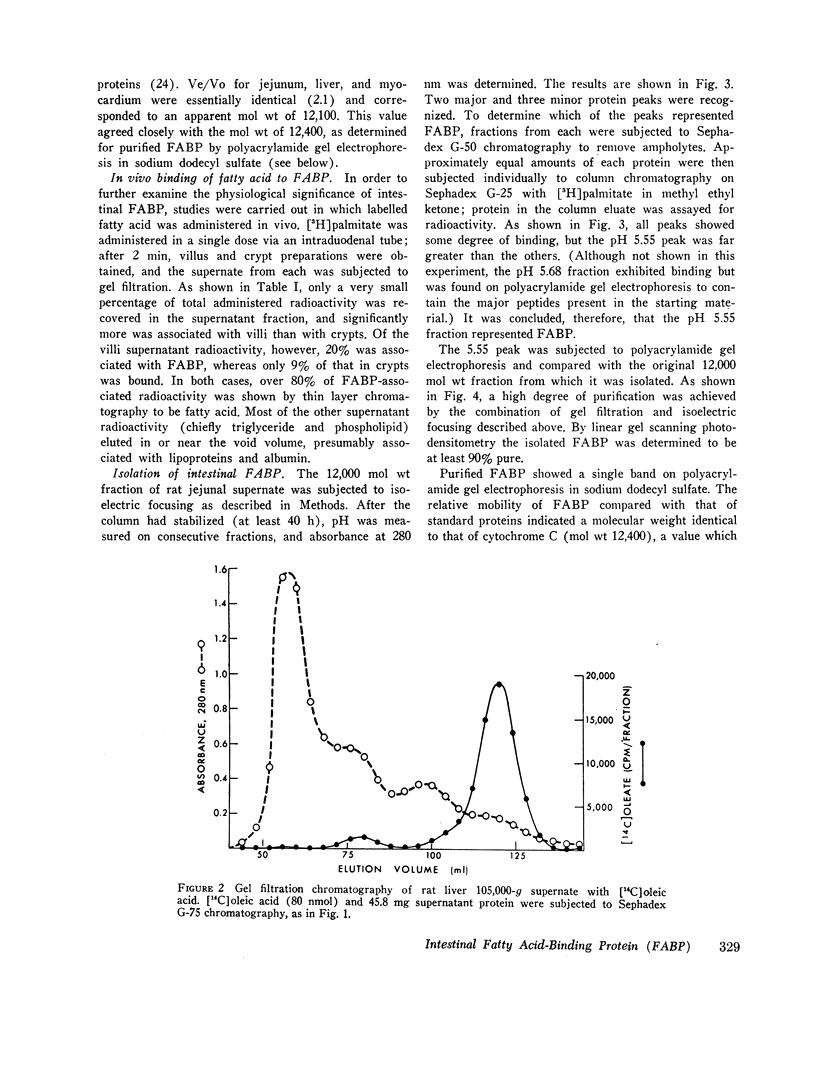
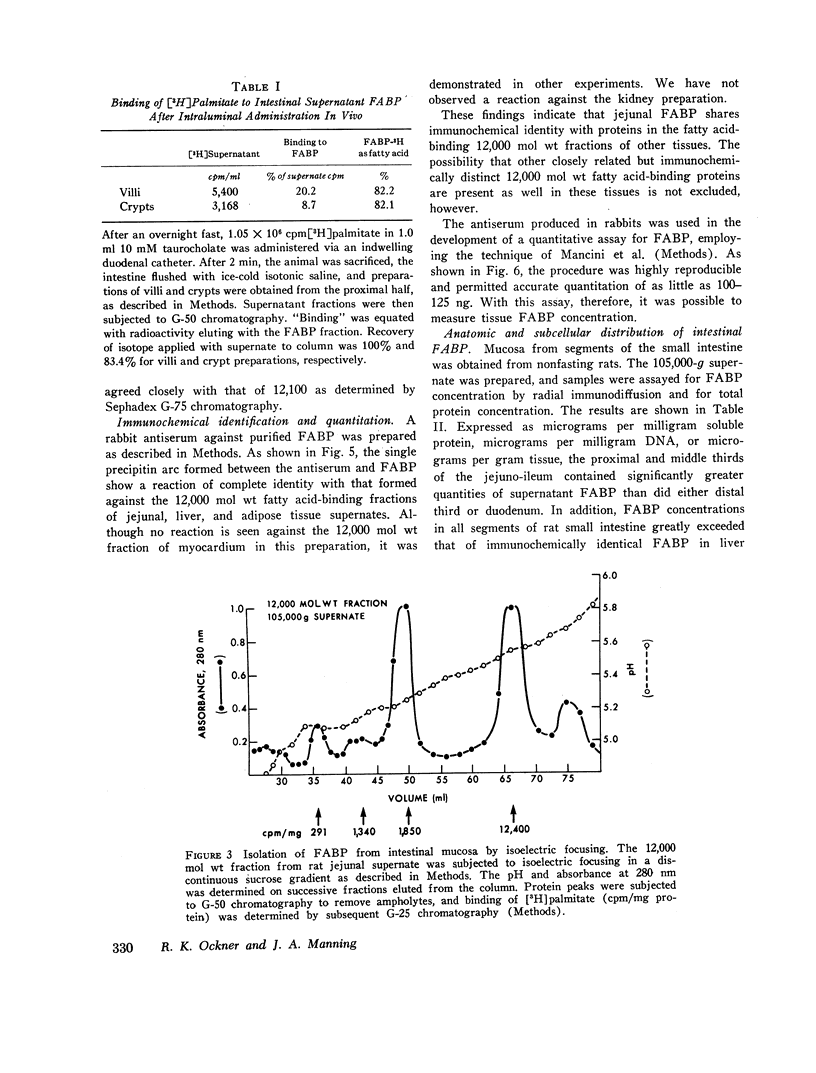
Abstract
A soluble fatty acid-binding protein (FABP), mol wt ∼ 12,000 is present in intestinal mucosa and other tissues that utilize fatty acids, including liver, myocardium, adipose, and kidney. This protein binds long chain fatty acids both in vivo and in vitro.
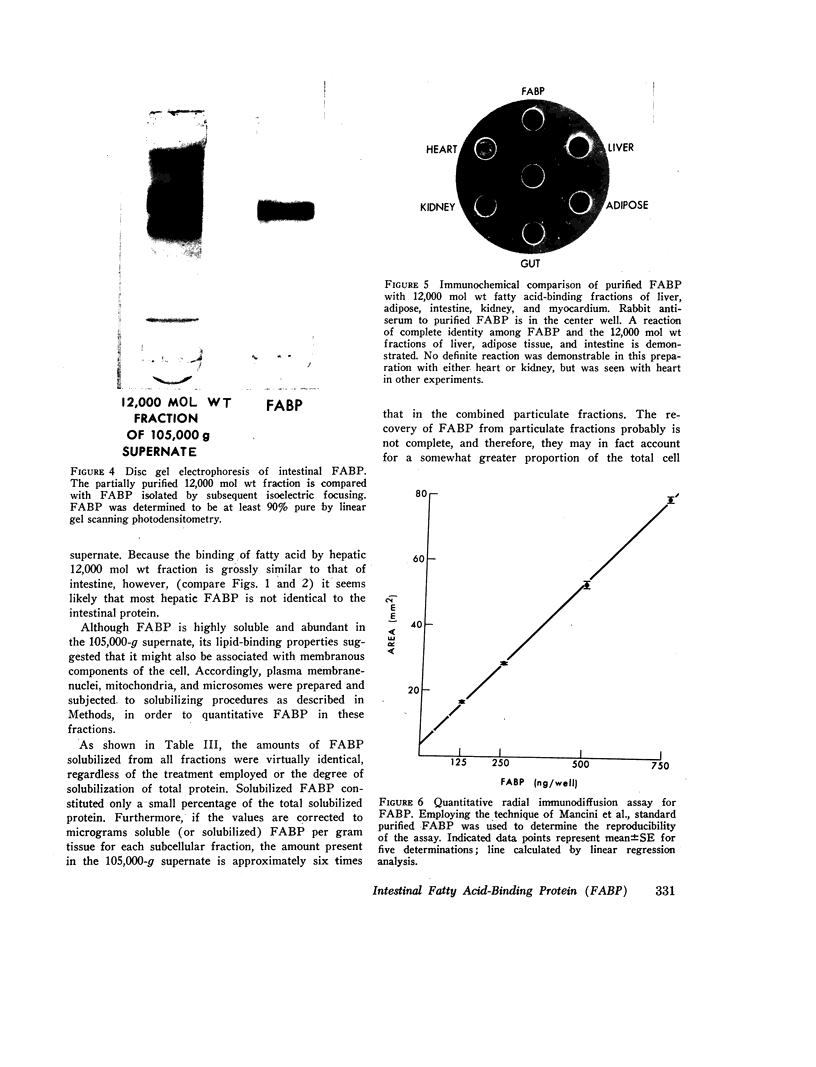
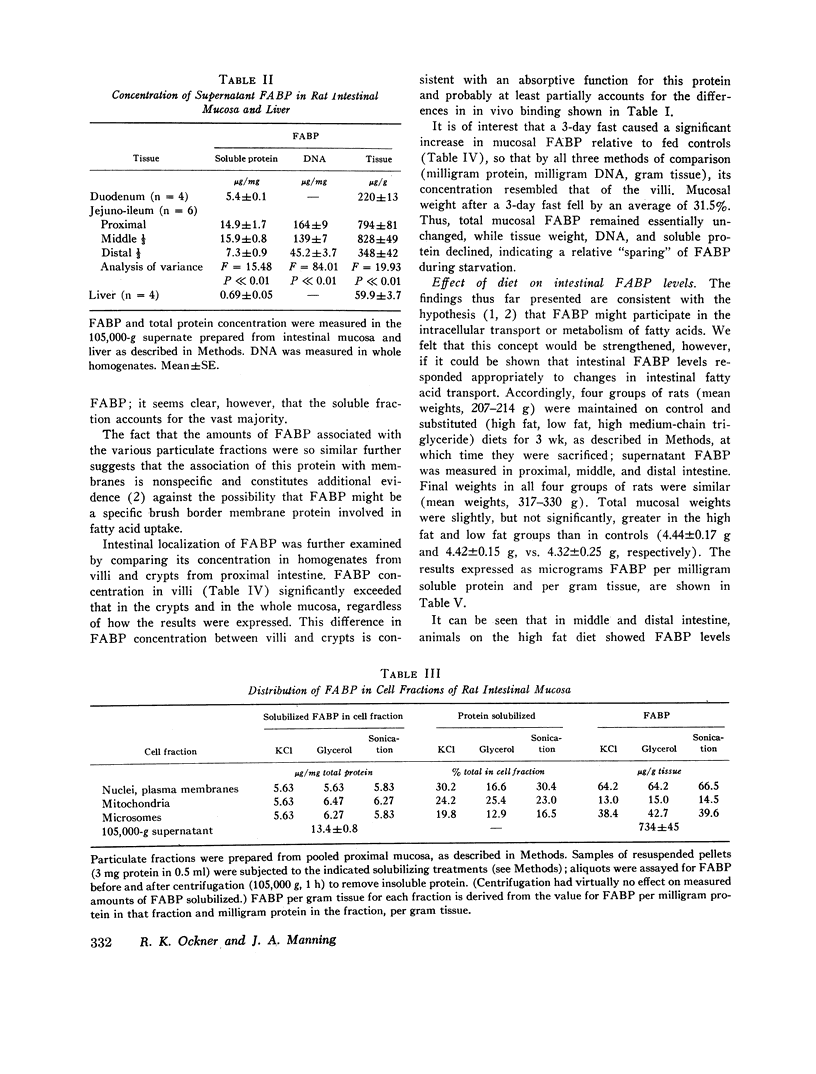
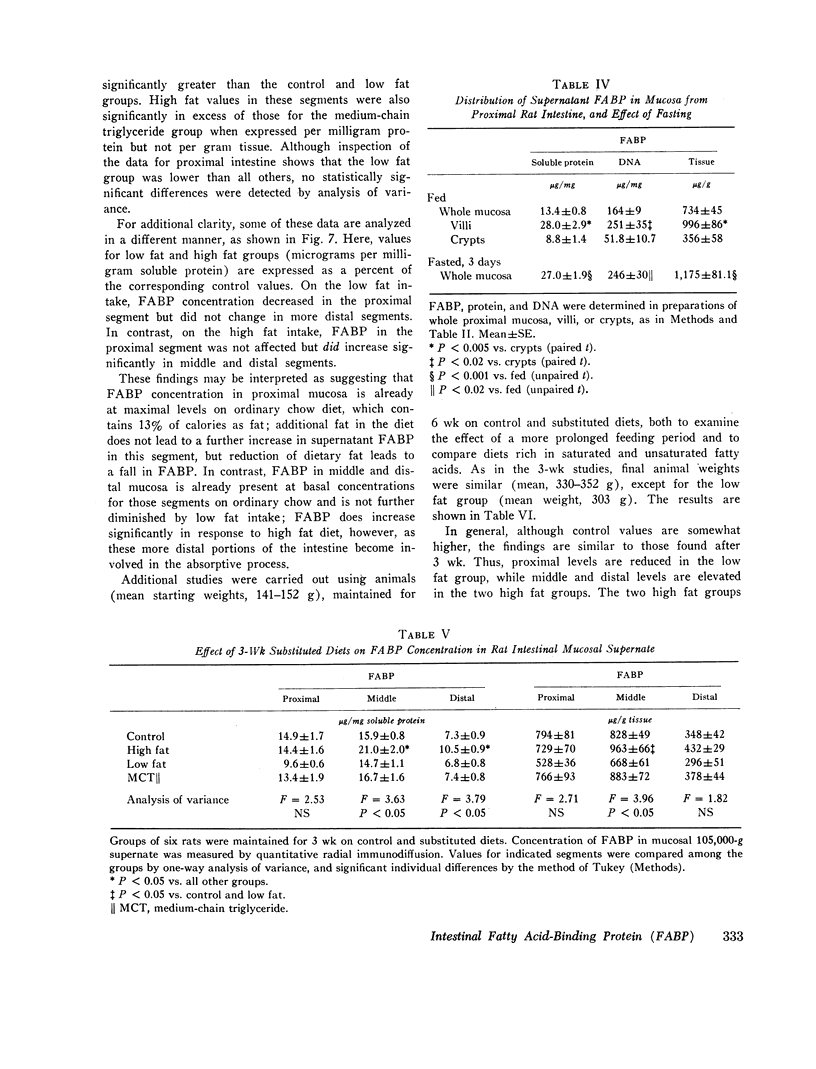
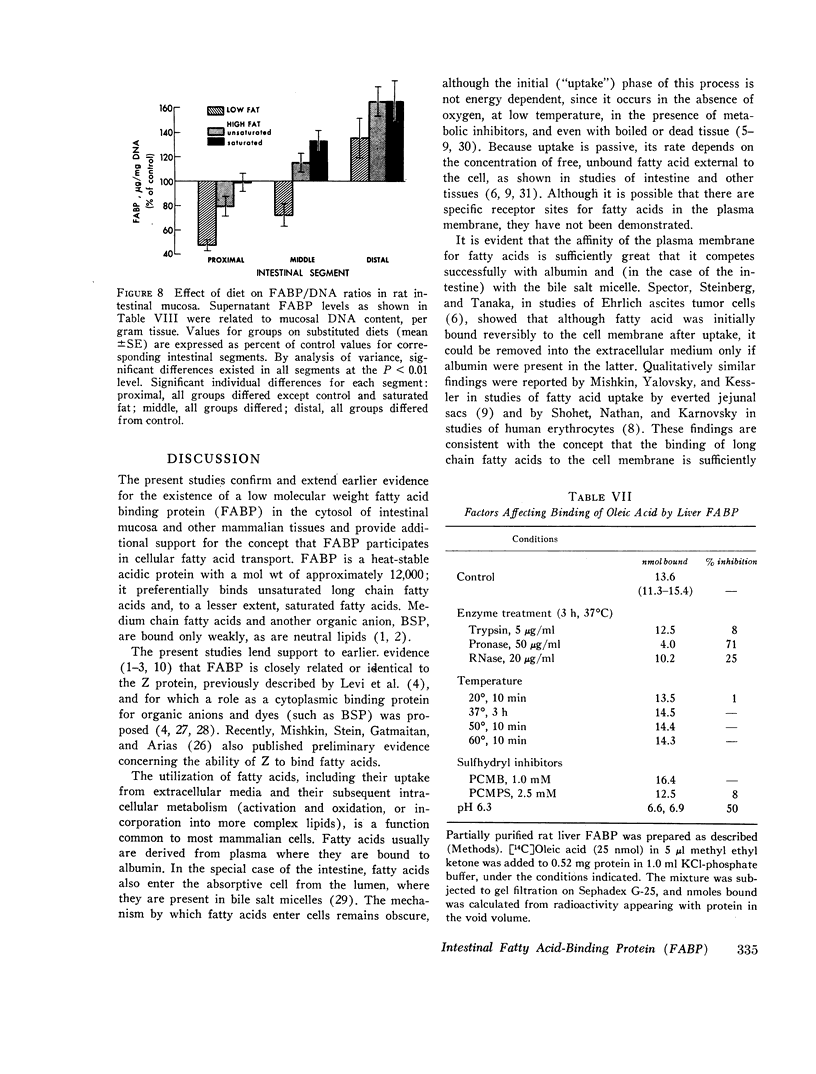
FABP was isolated from rat intestine by gel filtration and isoelectric focusing. It showed a reaction of complete immunochemical identity with proteins in the 12,000 mol wt fatty acid-binding fractions of liver, myocardium, and adipose tissue supernates. (The presence of immunochemically nonidentical 12,000 mol wt FABP in these tissues is not excluded.) By quantitative radial immunodiffusion, supernatant FABP concentration in mucosa from proximal and middle thirds of jejuno-ileum significantly exceeded that in distal third, duodenum, and liver, expressed as micrograms per milligram soluble protein, micrograms per gram DNA, and micrograms per gram tissue. FABP concentration in villi was approximately three times greater than in crypts. Small quantities of FABP were present in washed nuclei-cell membrane, mitochondrial and microsomal fractions. However, the amount of FABP solubilized per milligram membrane protein was similar for all particulate fractions, and total membrane-associated FABP was only about 16% of supernatant FABP. Intestinal FABP concentration was significantly greater in animals maintained on high fat diets than on low fat; saturated and unsaturated fat diets did not differ greatly in this regard.
The preponderance of FABP in villi from proximal and middle intestine, its ability to bind fatty acids in vivo as well as in vitro, and its response to changes in dietary fat intake support the concept that this protein participates in cellular fatty acid transport during fat absorption. Identical or closely related 12,000 mol wt proteins may serve similar functions in other tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H. Intestinal adaptation. N Engl J Med. 1973 Mar 8;288(10):520–521. doi: 10.1056/NEJM197303082881011. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- GOODMAN D. S. The interaction of human erythrocytes with sodium palmitate. J Clin Invest. 1958 Dec;37(12):1729–1735. doi: 10.1172/JCI103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN A. F., BORGSTROM B. Physico-chemical state of lipids in intestinal content during their digestion and absorption. Fed Proc. 1962 Jan-Feb;21:43–50. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Hoving J., Valkema A. J. Effect of dietary fat content on the site of fat absorption in hamster small intestine in vitro. Biochim Biophys Acta. 1969 Jul 29;187(1):53–58. doi: 10.1016/0005-2760(69)90132-5. [DOI] [PubMed] [Google Scholar]
- JOHNSTON J. M., BORGSTROEM B. THE INTESTINAL ABSORPTION AND METABOLISM OF MICELLAR SOLUTIONS OF LIPIDS. Biochim Biophys Acta. 1964 Aug 5;84:412–423. doi: 10.1016/0926-6542(64)90005-8. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kuhl W. E., Spector A. A. Uptake of long-chain fatty acid methyl esters by mammalian cells. J Lipid Res. 1970 Sep;11(5):458–465. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Deficiency of hepatic organic anion-binding protein, impaired organic amnion uptake by liver and "physiologic" jaundice in newborn monkeys. N Engl J Med. 1970 Nov 19;283(21):1136–1139. doi: 10.1056/NEJM197011192832104. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach C. M., 2nd Complex lipid synthesis in hamster intestine. Biochim Biophys Acta. 1973 Feb 14;296(2):386–402. doi: 10.1016/0005-2760(73)90097-0. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Stein L., Gatmaitan Z., Arias I. M. The binding of fatty acids to cytoplasmic proteins: binding to Z protein in liver and other tissues of the rat. Biochem Biophys Res Commun. 1972 Jun 9;47(5):997–1003. doi: 10.1016/0006-291x(72)90931-x. [DOI] [PubMed] [Google Scholar]
- Mishkin S., Yalovsky M., Kessler J. I. Stages of uptake and incorporation of micellar palmitic acid by hamster proximal intestinal mucosa. J Lipid Res. 1972 Mar;13(2):155–168. [PubMed] [Google Scholar]
- Ockner R. K., Manning J. A., Poppenhausen R. B., Ho W. K. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science. 1972 Jul 7;177(4043):56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Pittman J. P., Yager J. L. Differences in the intestinal absorption of saturated and unsaturated long chain fatty acids. Gastroenterology. 1972 May;62(5):981–992. [PubMed] [Google Scholar]
- Reyes H., Levi A. J., Gatmaitan Z., Arias I. M. Studies of Y and Z, two hepatic cytoplasmic organic anion-binding proteins: effect of drugs, chemicals, hormones, and cholestasis. J Clin Invest. 1971 Nov;50(11):2242–2252. doi: 10.1172/JCI106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
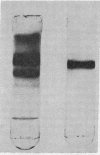
- Rodgers J. B., Tandon R., Fromm H. Acyl-CoA synthetase for long-chain fatty acids in rat small bowel and the influence of diets containing different compositions of fatty acids on intestinal lipid reesterifying enzyme activities. Biochim Biophys Acta. 1972 Aug 11;270(4):453–462. doi: 10.1016/0005-2760(72)90110-5. [DOI] [PubMed] [Google Scholar]
- Rosensweig N. S., Herman R. H. Control of jejunal sucrase and maltase activity by dietary sucrose or fructose in man. A model for the study of enzyme regulation in man. J Clin Invest. 1968 Oct;47(10):2253–2262. doi: 10.1172/JCI105910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Balint J. A., Edmonds R. H., Rodgers J. B. Adaptive changes of the rat small intestine in response to a high fat diet. Biochim Biophys Acta. 1972 Apr 18;260(4):708–715. doi: 10.1016/0005-2760(72)90019-7. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. The utilization of unesterified palmitate by Ehrlich ascites tumor cells. J Biol Chem. 1965 Oct;240(10):3747–3753. [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Sallee V. L., Dietschy J. M. Unstirred water layers in intestine: rate determinant of fatty acid absorption from micellar solutions. Science. 1971 Dec 3;174(4013):1031–1033. doi: 10.1126/science.174.4013.1031. [DOI] [PubMed] [Google Scholar]