Abstract
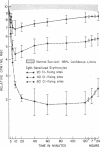
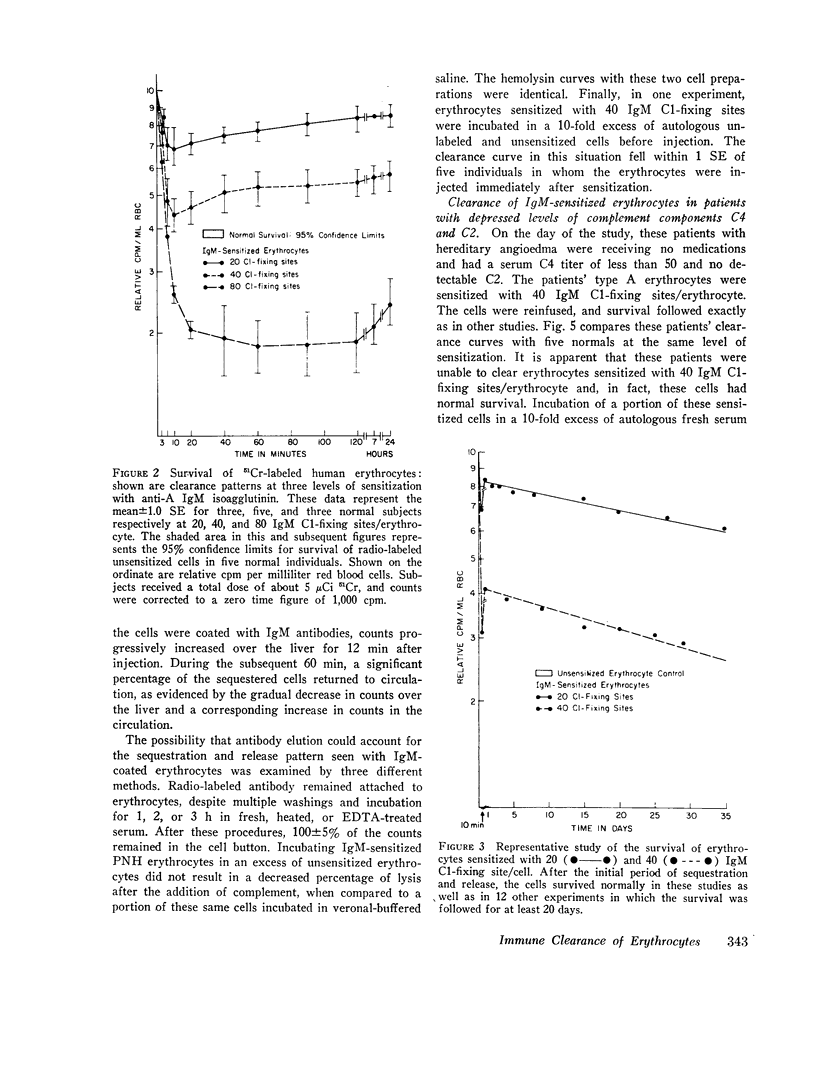
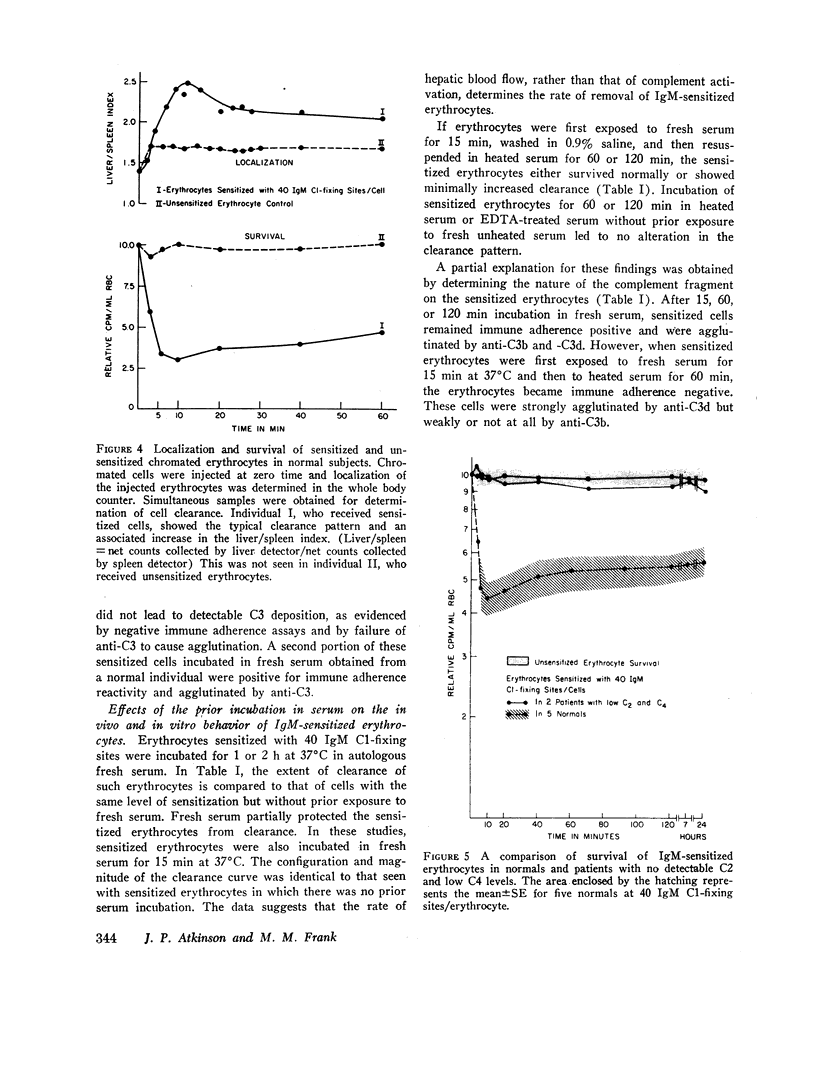
Purified human IgM isoagglutinins were utilized to sensitize 51Cr-labeled erythrocytes so as to produce a known number of complement-fixing sites. These cells were then reinfused into the erythrocyte donor. A minimum of 20 C1-fixing sites/erythrocyte were required for decreased survival. As the amount of antibody coating the erythrocytes was increased, a larger percentage was sequestered. With 80 C1-fixing sites, more than 75% of the injected erythrocytes were removed from the circulation within 10 min. In each case, the clearance pattern consisted of rapid hepatic sequestration followed by a gradual return of a portion of the erythrocytes into the circulation where they survived normally.
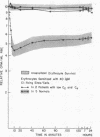
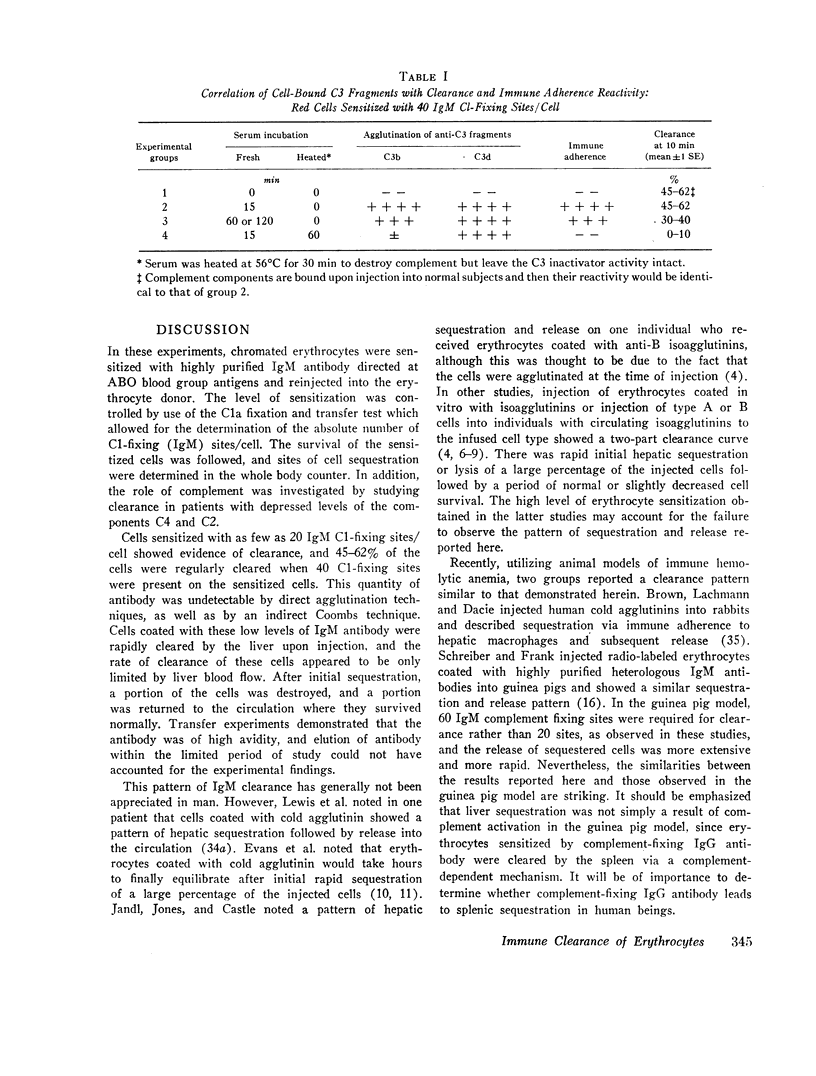
Clearance was shown to be dependent upon activation of the classical complement pathway, since sensitized cells survived normally in hereditary angioedema patients with low levels of C4 and no detectable C2. Exposure of sensitized cells to fresh serum for 15 min led to the deposition of 550-800 C3 molecules/C1-fixing site. Such cells were immune adherence positive, were agglutinated by anti-C3b, formed rosettes with human alveolar macrophages, and were sequestered in vivo, presumably because of the interaction of cell-bound C3b with the C3b receptor on hepatic macrophages. After exposure to heated serum as a source of the C3b inactivator, the cells were immune adherence negative, were agglutinated only by anti-C3d, did not form rosettes with macrophages, and survived normally in vivo despite, being Coombs positive. Cleavage of cell-bound C3b to C3d may explain the release phase of the IgM clearance pattern. Whereas erythrocytes coated with IgM antibody and complement were previously thought to be sequestered in the liver because of extensive membrane damage, these experiments suggest that clearance is determined by the interaction of erythrocyte-bound complement fragments with specific receptors on hepatic macrophages.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Lo Buglio A. F., Jandl J. H., Cotran R. S. The interaction between human monocytes and red cells. Binding characteristics. J Exp Med. 1970 Dec 1;132(6):1191–1206. doi: 10.1084/jem.132.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews H. L., Peterson D. C., Murphy R. E., Myers E. J. An organic plastic, localizing whole-body counter. J Nucl Med. 1965 Sep;6(9):667–678. [PubMed] [Google Scholar]
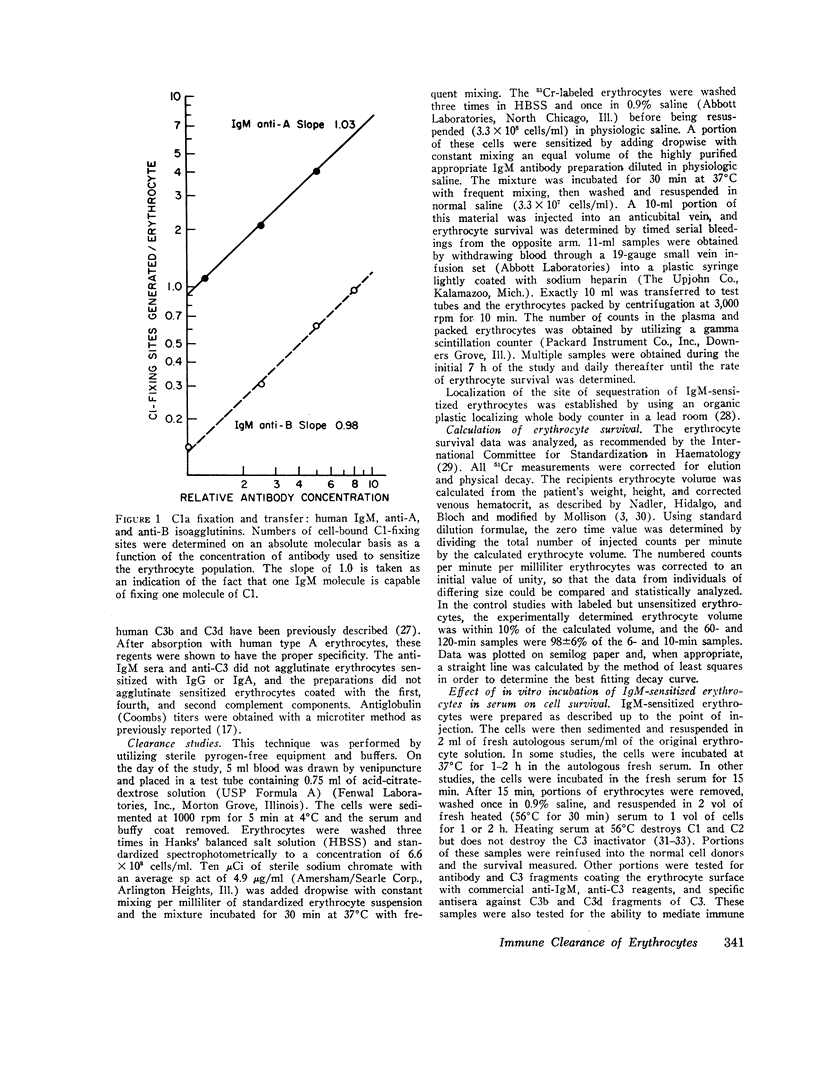
- Borsos T., Colten H. R., Spalter J. S., Rogentine N., Rapp H. J. The C'la fixation and transfer test: examples of its applicability to the detection and enumeration of antigens and antibodies at cell surfaces. J Immunol. 1968 Sep;101(3):392–398. [PubMed] [Google Scholar]
- Borsos T., Leonard E. J. Detection of bound C3 by a new immunochemical methods. J Immunol. 1971 Sep;107(3):766–771. [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Brown D. L., Lachmann P. J., Dacie J. V. The in vivo behaviour of complement-coated red cells: studies in C6-deficient, C3-depleted and normal rabbits. Clin Exp Immunol. 1970 Sep;7(3):401–421. [PMC free article] [PubMed] [Google Scholar]
- CUTBUSH M., MOLLISON P. L. Relation between characteristics of blood-group antibodies in vitro and associated patterns of redcell destruction in vivo. Br J Haematol. 1958 Apr;4(2):115–137. doi: 10.1111/j.1365-2141.1958.tb03843.x. [DOI] [PubMed] [Google Scholar]
- Dacie J. V. Autoimmune haemolytic anaemias. Br Med J. 1970 May 16;2(5706):381–386. doi: 10.1136/bmj.2.5706.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelfriet C. P., Pondman K. W., Wolters G., Borne AE von dem, Beckers D., Misset-Groenveld G., van Loghem J. J. Autoimmune haemolytic anaemias. 3. Preparation and examination of specific antisera against complement components and products, and their use in serological studies. Clin Exp Immunol. 1970 May;6(5):721–732. [PMC free article] [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M. Chronic hemolytic anemia due to cold agglutinins: the mechanism of resistance of red cells to C' hemolysis by cold agglutinins. J Clin Invest. 1967 Sep;46(9):1461–1474. doi: 10.1172/JCI105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. S., Turner E., Bingham M., Woods R. Chronic hemolytic anemia due to cold agglutinins. II. The role of C' in red cell destruction. J Clin Invest. 1968 Apr;47(4):691–701. doi: 10.1172/JCI105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Dourmashkin R. R., Humphrey J. H. Observations on the mechanism of immune hemolysis: importance of immunoglobulin class and source of complement on the extent of damage. J Immunol. 1970 Jun;104(6):1502–1510. [PubMed] [Google Scholar]
- Frank M. M., Gaither T. Evidence that rabbit gamma G haemolysin in capable of utilizing guinea-pig complement more efficiently than rabbit gamma M haemolysin. Immunology. 1970 Dec;19(6):975–981. [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Gaither T. The effect of temperature on the reactivity of guinea-pig complement with gamma G and gamma M haemolytic antibodies. Immunology. 1970 Dec;19(6):967–974. [PMC free article] [PubMed] [Google Scholar]
- Frank M. M., Sergent J. S., Kane M. A., Alling D. W. Epsilon aminocaproic acid therapy of hereditary angioneurotic edema. A double-blind study. N Engl J Med. 1972 Apr 13;286(15):808–812. doi: 10.1056/NEJM197204132861503. [DOI] [PubMed] [Google Scholar]
- Gaither T. A., Frank M. M. Studies of complement-mediated membrane damage: the influence of erythrocyte storage on susceptibility to cytolysis. J Immunol. 1973 Feb;110(2):482–489. [PubMed] [Google Scholar]
- Huber H., Douglas S. D. Receptor sites on human monocytes for complement: binding of red cells sensitized by cold autoantibodies. Br J Haematol. 1970 Jul;19(1):19–26. doi: 10.1111/j.1365-2141.1970.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Huber H., Polley M. J., Linscott W. D., Fudenberg H. H., Müller-Eberhard H. J. Human monocytes: distinct receptor sites for the third component of complement and for immunoglobulin G. Science. 1968 Dec 13;162(3859):1281–1283. doi: 10.1126/science.162.3859.1281. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., JONES A. R., CASTLE W. B. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957 Oct;36(10):1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES N. C., MOLLISON P. L., VEALL N. Removal of incompatible red cells by the spleen. Br J Haematol. 1957 Apr;3(2):125–133. doi: 10.1111/j.1365-2141.1957.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- LoBuglio A. F., Cotran R. S., Jandl J. H. Red cells coated with immunoglobulin G: binding and sphering by mononuclear cells in man. Science. 1967 Dec 22;158(3808):1582–1585. doi: 10.1126/science.158.3808.1582. [DOI] [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Adams J. P. Complement-dependent immune adherence measured with human granulocytes: changes in the antigenic nature of red cell-bound C3 produced by incubation in human serum. Clin Immunol Immunopathol. 1973 Apr;1(3):398–407. doi: 10.1016/0090-1229(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Adams J. P. Mechanisms of immune lysis of red blood cells in vitro. I. Paroxysmal nocturnal hemoglobinuria cells. J Clin Invest. 1973 May;52(5):1129–1137. doi: 10.1172/JCI107279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue G. L., Rosse W. F., Gockerman J. P. Measurement of the third component of complement bound to red blood cells in patients with the cold agglutinin syndrome. J Clin Invest. 1973 Feb;52(2):493–501. doi: 10.1172/JCI107206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLISON P. L. Blood-group antibodies and red-cell destruction. Br Med J. 1959 Nov 21;2(5159):1035–1041. doi: 10.1136/bmj.2.5159.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., Rabinovitch M., Nussenzweig V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J Exp Med. 1972 Apr 1;135(4):780–792. doi: 10.1084/jem.135.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. E., Frank M. M. Complement-mediated tissue damage: contribution of the classical and alternate complement pathways in the Forssman reaction. J Immunol. 1972 Jun;108(6):1517–1525. [PubMed] [Google Scholar]
- May J. E., Green I., Frank M. M. The alternate complement pathway in cell damage: antibody-mediated cytolysis of erythrocytes and nucleated cells. J Immunol. 1972 Sep;109(3):595–601. [PubMed] [Google Scholar]
- Mollison P. L. The role of complement in antibody-mediated red-cell destruction. Br J Haematol. 1970 Mar;18(3):249–255. doi: 10.1111/j.1365-2141.1970.tb01440.x. [DOI] [PubMed] [Google Scholar]
- Petz L., Fudenberg H. H., Fink D. Immune adherence reactions of human erythrocytes sensitized with complement in vitro and in vivo. J Immunol. 1971 Dec;107(6):1714–1722. [PubMed] [Google Scholar]
- Reesink H. W., van der Hart M., van Loghem J. J. Evaluation of a simple method for determination of IgG titre anti-A or-B in cases of possible ABO blood group incompatibility. Vox Sang. 1972;22(5):397–407. doi: 10.1111/j.1423-0410.1972.tb03987.x. [DOI] [PubMed] [Google Scholar]
- Rifkind R. A. Destruction of injured red cells in vivo. Am J Med. 1966 Nov;41(5):711–723. doi: 10.1016/0002-9343(66)90032-5. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972 Mar;51(3):575–582. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. D., Frank M. M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Invest. 1972 Mar;51(3):583–589. doi: 10.1172/JCI106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C., Davis N. C., Forristal J., Herbst J., Spitzer R. Antigenic determinants of human beta-1c and beta-1g-globulins. J Immunol. 1966 Apr;96(4):650–658. [PubMed] [Google Scholar]