Abstract
Background:
We recently implemented the use of an ex-vivo porcine model to teach residents the fundamentals of performing a laparoscopic Nissen fundoplication.
Methods:
Residents were trained using intact porcine esophagus, stomach, and spleen placed in a standard video-trainer. They were later asked to complete a survey containing a course evaluation.
Results:
Sixteen residents (R1-R4) completed the survey. They agreed that (1) the exercise was a valuable use of their limited time, (2) repeating the exercise will be of additional benefit, (3) it will improve their ability to perform or assist in an actual case in the OR, and (4) the surgical principles learned using the model will transfer to other laparoscopic cases. Significant subjective improvements were reported in resident comfort level in assisting in or performing a laparoscopic Nissen fundoplication.
Conclusions:
The use of an inexpensive ex-vivo porcine training model increases resident comfort level in performing a Nissen fundoplication in the operating room.
Keywords: Laparoscopic drills, Surgical skills training, Laboratory setting, Resident education, Nissen fundoplication
INTRODUCTION
Laparoscopic skills training in the laboratory setting is becoming increasingly popular. Before the implementation of the 80-hour workweek, residents reported an inadequate exposure to advanced laparoscopic cases.1 Diminished time in the hospital may lead to further inadequacy in advanced laparoscopic training. Therefore, in an effort to better train residents in the technically demanding field of laparoscopy, we implemented the use of an inexpensive ex-vivo porcine training model as a part of our laparoscopic skills laboratory-training curriculum. Subsequently, we surveyed residents to evaluate the perceived benefit of the laboratory session.
METHODS
Study Population and Design
Residents were scheduled to attend the laboratory training sessions as a part of a structured training curriculum in the surgical skills laboratory. Residents of all training levels in the General Surgery Residency program, both categorical and preliminary, were trained using this model. Training sessions occurred on Monday afternoons from 2:00 pm to 5:00 pm over a period of 8 weeks, with 6 residents scheduled for each session.
Institutional Animal Care and Use Committee (IACUC) approval was obtained and intact porcine esophagus, stomach, and spleen were purchased from a local meat vendor at a cost of $4.00 USD each. Organs were suspended from a standard test-tube clamp on a ring stand inside of a nontransparent box fitted with trocar ports for insertion of instruments (Figure 1). Standard laparoscopic equipment was utilized, including a camera with a 10-mm 0°-telescope, a high definition 19-inch monitor, and a light source.
Figure 1.
(A) Laparoscopic video trainer, and (B) ex-vivo porcine organ setup.
The training session consisted of distribution of written material on laparoscopic Nissen fundoplication including anatomy, operative procedure, and complications. This was followed by a demonstration of the technique of laparoscopic Nissen fundoplication by an attending surgeon, with emphasis placed on the fundamental aspects of advanced laparoscopic surgery including the use of both intra- and extracorporeal knot tying as well as the use of the Harmonic scalpel (Figure 2). Additionally, procedure-specific emphasis was placed on dissection and ligation of the short gastric vessels to mobilize the fundus and subsequent creation of a retroesophageal space with passage of the fundus behind the esophagus and creation of a loose 360-degree fundoplication. Following the demonstration, residents were allowed to attempt fundoplication on a fresh organ set with unlimited opportunity to ask questions of the attending surgeon.
Figure 2.
(A) Demonstration of the laparoscopic Nissen fundoplication, including (B) intracorporeal knot tying.
Survey Technique
Residents were surveyed retrospectively 6 months following the training to better evaluate the long-term opinion of the laboratory session (Figure 3). Resident survey identity was kept confidential from the department by laboratory staff to allow follow-up questioning while ensuring honest responses. Survey demographic data included training level, categorical status, and prior surgical experience. Laboratory evaluation data included value of time spent in the laboratory, transferability of skills to the operating room, realism, difficulty, overall laparoscopic skill set value, and desire to repeat the exercise. Additionally, residents were surveyed for both pre- and posttraining knowledge level and comfort level in assisting and performing a laparoscopic Nissen fundoplication.
Figure 3.
Posttraining resident survey.
Statistical Methods
Mean resident subjective ratings of pre- and posttraining (1) knowledge of the procedure, (2) comfort assisting with the procedure, and (3) comfort performing the procedure as the primary surgeon were treated as ordinal variables for all analyses. Means, standard deviations, and number of observations were presented for each variable. The experimental unit was each individual subject (n=16). The experiment used a repeated measures design with each subject's response evaluated at 2 periods (pre- and posttraining). The null hypothesis was that there would be no difference between periods. To apply ANOVA methods, a “normalized-rank” transformation was applied to the data. The rank-transformed data was analyzed by using a mixed-model ANOVA for repeated measures. Differences between pre- and posttraining ratings (rejection of the null hypothesis) were considered significant if the probability of chance occurrence was <0.05 using 2-tailed tests.
RESULTS
Study Population Demographics
Twenty residents received laboratory training. Survey data were collected from 16 (80%) residents. Four residents are no longer in the General Surgery Residency training program, and therefore their follow-up data were unobtainable. Surveys were returned between 6 and 12 months following training. Training level ranged from R1 to R4 (Mean postgraduate year = 1.8: 9 R1, 2 R2, 4 R3, 1 R4). Twelve (75%) participants were categorical General Surgery Residents; 4 were Preliminary Surgery Residents.
Survey Data
Prior Nissen fundoplication and laparoscopic surgical experience is depicted in Figure 4. Mean rating (scale 1–5) of (A) overall improvement in laparoscopic skill set, (B) difficulty level, (C) realism of exercise, (D) transferability to the operating room, (E) was it a valuable use of their time, and (F) desire to repeat the exercise are depicted in Figure 5. All residents reported that participating in the exercise was a valuable use of their time, that it was appropriately difficult, and that the skills acquired will transfer to the operating room. Fifteen (94%) residents reported that the exercise added to their overall laparoscopic skill set and that repeating the exercise would be of further benefit. Fourteen (88%) residents felt that the model was realistic, with 2 residents upon post-hoc questioning citing the lack of bleeding tissue as a problem. Significant subjective improvements in mean pre- versus posttraining—(1) knowledge level (3.9 vs 6.4, P<0.0001), (2) comfort assisting with a laparoscopic Nissen fundoplication (3.8 vs 6.1, P<0.0001), and (3) comfort performing a laparoscopic Nissen fundoplication as the primary surgeon (2.7 vs 4.2, P<0.0002) are depicted in Figure 6.
Figure 4.

Prior resident laparoscopic cholecystectomy, laparoscopic appendectomy, open Nissen fundoplication, and laparoscopic Nissen fundoplication experience as an observer, first assistant, and primary surgeon.
Figure 5.
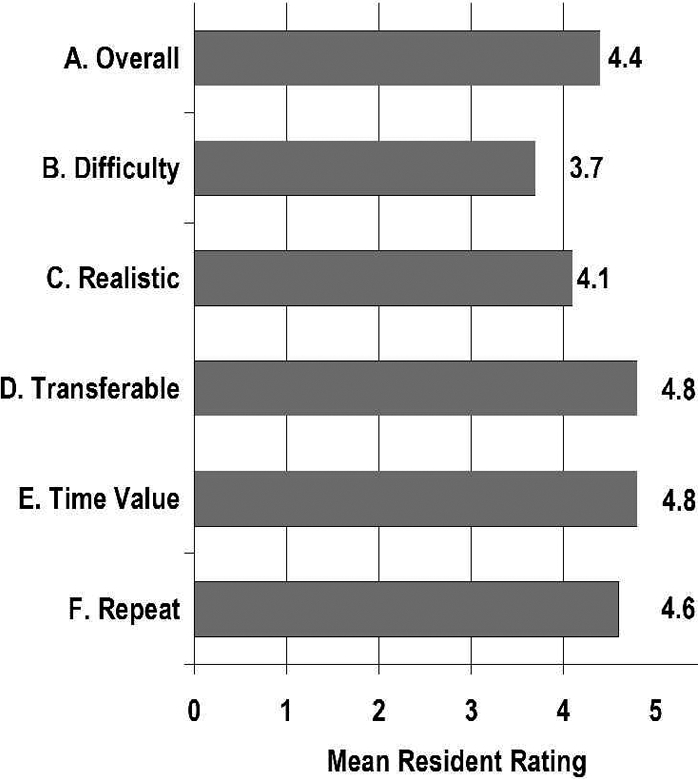
Subjective resident rating of (A) overall improvement in laparoscopic skill set, (B) difficulty level, (C) realism of exercise, (D) transferability to the operating room, (E) time value, and (F) desire to repeat exercise (scale 1–5, see Figure 3)
Figure 6.

Subjective pre- vs posttraining resident knowledge level, comfort assisting with a laparoscopic Nissen fundoplication, and comfort performing a laparoscopic Nissen fundoplication as the primary surgeon (Mean ± SEM, P<0.0002)
DISCUSSION
Laparoscopy has become a major component of general surgery and therefore of general surgery residency training. Residents have reported an inadequate exposure to advanced laparoscopic cases, with an expectation of completing fewer than 4 to 7 laparoscopic Nissen fundoplications by the end of their general surgical residency.1 In the same study, residents report the need to complete at least 10 cases before being proficient at the procedure.1 A retrospective case review has shown an increased rate of conversion to laparotomy in the initial 30 cases and an increased operative time and hospital stay in the first 26 cases.2 Additionally, Watson et al3 demonstrated that the learning curve for fundoplication is as high as 20 cases depending on the experience of the individual supervisor and of the institution in general. Furthermore, a recent meta-analysis4 of 11 studies (1558 patients) evaluating operative time, conversion rate, complication rate, and hospital stay revealed a mean of 28 cases (range, 20 to 60) to attain proficiency in laparoscopic fundoplication. A study5 from a community program suggests that residents can be exposed to an adequate number of cases to be trained in the operating room alone in a small training program. Academic medical centers have implemented the use of full-time minimally invasive surgeons to train their faculty colleagues in advanced laparoscopic surgery, resulting in a department-wide increase in advanced laparoscopic cases.6 An alternative to increasing operating room exposure is early laboratory bench model exposure, which may transfer the early portion of the learning curve out of the operating room. Major barriers to becoming proficient in advanced laparoscopic surgery include the grasp of working in a 3-dimensional space under 2-dimensional visualization and the mastering of laparoscopic suturing.7 For the practicing surgeon inexperienced in minimally invasive surgery, operative skills under 2-dimensional visualization can be improved using less technically challenging procedures, such as laparoscopic appendectomy and laparoscopic cholecystectomy. However, the acquisition of advanced laparoscopic skills in the apprenticeship environment can be cost-prohibitive due to time spent away from the practice and cost of hiring an experienced endoscopic surgeon to participate in training.8 Furthermore, fundamental skills like tissue handling and efficiency of motion, as studied by Richards et al8 using force/torque signatures, have been show to improve with experience. Inexperienced surgeons tend to exert too much pressure on tissues being manipulated, risking injury, and too little pressure while dissecting, suggesting hesitancy.9 Perhaps, if the fundamental skills of intracorporeal suturing, the use of the Harmonic scalpel, comfort working under 2-dimensional visualization, familiarity with handling tissues using endoscopic grasping devices, and economy of motion were mastered before embarking on actual human fundoplication, the time and monetary cost of operating room training could be reduced.
Many modalities of laparoscopic training outside of the operating room have been described, each of which carries its own advantages and disadvantages. Multimedia interactive programs carry the major advantage of familiarizing the resident with the anatomy, fundamentals, and steps of the procedure without the requirement of specialized equipment other than a standard personal computer.10 However, these programs do not effectively develop the technical skills required to perform advanced laparoscopic surgery. The ex-vivo model we are proposing takes advantage of real tissue to develop technical skills, while depending on a didactic session to develop the knowledge base of the trainee. An alternative to an ex-vivo model is the use of an intact porcine model. Although the living model has many advantages, including nondevitalized tissue that bleeds, the cost of this model is significant. At our institution, the combined cost of the animal, veterinarian, and operating room time is approximately $1,000.00 USD per animal. Although we do utilize the intact porcine model as a part of our training program (approximately 12 animals per year), the associated cost prohibits junior residents from performing advanced laparoscopic procedures as these portions are reserved for the senior residents. In contrast, the combined cost of 7 (1 attending, 6 trainee) organ sets is $128.00 USD ($4.00 per organ plus approximately a $100.00 delivery fee). Ordering organs in bulk can minimize the impact of the delivery fee because organs are easily frozen and thawed at a later date for use in the laboratory. The ease of organ storage and minimal cost of the ex-vivo model allows an unlimited number of repetitions, which familiarize residents with fundamental skills before they undertake a fundoplication on a live animal. The ideal laparoscopic skills training program will incorporate multiple training modalities in a progressively structured curriculum that takes advantage of the benefits of each successive modality.
Overall, the resident response to the ex-vivo porcine training model has been significantly positive. Junior residents have the opportunity to acquire fundamental skills before attempting a Nissen fundoplication in the operating room. Additionally, senior residents can refine their skills, focusing on areas of the procedure they perceive as weaknesses, achieving further independent technical proficiency. The laboratory provides a setting in which trainees can ask questions and receive feedback without the time and cost constraints of the operating room. Furthermore, mistakes such as gastrotomy or esophageal perforation do not carry the same consequence as they do in actual patients, allowing the training of more inexperienced first-year residents without risk of patient injury. Furthermore, the ease of storage and minimal cost of the organs provides ample opportunity for residents to repeat the exercise an unlimited number of times until they feel proficient. The combination of all of these factors may lead to a faster and shallower learning curve in the operating room.
A major weakness of this study is the subjective nature of the data. Follow-up objective studies will be required to validate this initial experience. Randomized, controlled observation of initial resident fundoplication in the operating room of those residents receiving laboratory training compared with those not receiving training will be the definitive measure of the efficacy of this model. Additionally, such a study could validate the hypothesis that the early portion of the learning curve can be shifted to the bench laboratory. An additional weakness is the lack of an adequate number of senior residents in the sample. Further study will be required to ascertain the benefit of the laboratory for residents with prior laparoscopic fundoplication experience.
CONCLUSION
The use of an inexpensive ex-vivo porcine training model increases resident comfort level in performing a Nissen fundoplication in the operating room. In addition, residents report that it is a valuable educational tool, improving their overall laparoscopic skill set. Finally, residents report the desire to return to the laboratory and repeat the exercise, verifying that it is a subjectively valuable use of their time.
Footnotes
Presented at the 13th Annual Congress for Endosurgery in Children (IPEG). Maui, Hawaii, USA, May 6 – 8, 2004.
Contributor Information
Aaron R. Jensen, Department of Surgery, Temple University School of Medicine, Philadelphia, Pennsylvania, USA.
Richard Milner, Department of Surgery, Temple University School of Medicine, Philadelphia, Pennsylvania, USA.
John Gaughan, Department of Biostatistics, Temple University School of Medicine, Philadelphia, Pennsylvania, USA.
Harsh Grewal, Department of Surgery, Temple University School of Medicine, Philadelphia, Pennsylvania, USA; Section of Pediatric Surgery, Temple University Children's Medical Center Philadelphia, Pennsylvania, USA.
References:
- 1. Rattner DW, Apelgren KN, Eubanks WS. The need for opportunities in advanced laparoscopic surgery: the residents' perspective. Surg Endosc. 2001;15:1066–1070 [DOI] [PubMed] [Google Scholar]
- 2. Deschamps C, Allen MS, Trastek VF, Johnson JO, Pairolero PC. Early experience and learning curve associated with laparoscopic Nissen fundoplication. J Thorac Cardiovasc Surg. 1998; 115:281–285 [DOI] [PubMed] [Google Scholar]
- 3. Watson DI, Baigrie RJ, Jamieson GG. A learning curve for laparoscopic fundoplication: Definable, avoidable, or a waste of time? Ann Surg. 1996;224:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dagash H, Chowdhury M, Pierro A. When can I become proficient in laparoscopic surgery? A systematic review of the evidence. J Pediatr Surg. 2003;38:720–724 [DOI] [PubMed] [Google Scholar]
- 5. Reynolds FD, Goudas L, Zuckerman RS, Gold MS, Heneghan S. A rural, community-based program can train residents in advanced laparoscopy. J Am Coll Surg. 2003;197:620–623 [DOI] [PubMed] [Google Scholar]
- 6. Fowler DL, Hogle N. The impact of a full-time director of minimally invasive surgery: Clinical practice, education, and research. Surg Endosc. 2000;14:444–447 [DOI] [PubMed] [Google Scholar]
- 7. Watson DI, Jamieson GG, Baigrie RJ, et al. Laparoscopic surgery for gastro-oesophageal reflux: Beyond the learning curve. Br J Surg. 1996;83:1284–1287 [PubMed] [Google Scholar]
- 8. Chang JHT, Rothenberg SS, Bealer JF, Hamby LA, Suadi RW. Endosurgery and the senior pediatric surgeon. J Pediatr Surg. 2001;36:690–692 [DOI] [PubMed] [Google Scholar]
- 9. Richards C, Rosen J, Hannaford B, Pellegrini C, Sinanan M. Skills evaluation in minimally invasive surgery using force/ torque signatures. Surg Endosc. 2000;14:791–798 [DOI] [PubMed] [Google Scholar]
- 10. Ramshaw BJ, Young D, Garcha I, et al. The role of multimedia interactive programs in training for laparoscopic procedures. Surg Endosc. 2001;15:21–27 [DOI] [PubMed] [Google Scholar]





