Abstract
Background:
Gastric electrical stimulation has been proven effective for drug-refractory gastroparesis. Placement of stimulator leads and device usually requires a laparotomy, although laparoscopic placement has also been used.
Methods:
To compare laparotomy with laparoscopy, we examined 36 patients, 18 undergoing laparoscopy and 18 undergoing laparotomy, matched for primary diagnosis and health resource usage. We compared baseline symptoms, length of surgery, length of postoperative hospital stay, gastric emptying, and health resources in each of the 2 groups over time, to see what variables, if any, differed.
Results:
Baseline symptoms, gastric emptying, and health resource usage were similar. Operative times were also similar, but length of stay declined from a mean of 6.4 days for laparotomy to 1.1 days for laparoscopy. Long-term outcome, via symptoms, gastric emptying, and health resource utilization were comparable between the 2 groups.
Conclusion:
Laparoscopic placement of gastric electrical stimulator leads and device is associated with shorter lengths of postoperative hospital stay. However, the patients who underwent laparotomy had higher vomiting scores and more previous abdominal surgeries at baseline, and higher long-term mortality at follow-up, suggesting that they may be more ill, as a group, than the laparoscopic patients. Laparoscopic placement of devices may be preferable when technically feasible.
Keywords: Gastroparesis, Laparoscopy, Laparotomy
INTRODUCTION
Gastroparesis, a diverse disorder manifesting with symptoms of upper gut dysfunction/severe dyspepsia and disordered gastric emptying, can be difficult to treat. The most commonly recognized cause for gastroparesis is the neuropathy of long-term diabetes mellitus, although the cause in many patients is of idiopathic or postsurgical origin.1 The lack of effective gastro-kinetic drugs and the loss of several drugs previously available has driven a need for novel therapies. Patients with severe gastroparesis often suffer repeated hospitalizations, poor quality of life, and many complications from the few available therapies. Gastric electrical stimulation, which was introduced in this form in January 1992 in Memphis Tennessee, has recently been studied in a series of initial worldwide trials. Gastric electrical stimulation (GES) has been shown to be effective for drug-refractory gastroparesis (GP), as evidenced by a recent double-blind, randomized, placebo-controlled study, and at 2 open label trials.2,3 However, placement of a permanent GES device, since its inception in the early 1990s, traditionally requires a laparotomy. The introduction of laparoscopy offers the potential for shorter hospitalizations, but it has not been clear whether the long-term outcomes were equivalent to outcomes for laparoscopy. We compared the operative times (OT), length of stay (LOS), and long-term outcomes of 18 consecutive patients undergoing laparoscopic GES placement (Lap) matched with 18 patients undergoing GES placement by laparotomy (Open).
METHODS
Eighteen consecutive patients who had GP causes of diabetes mellitus, postsurgical and idiopathic disease underwent laparoscopic (Lap) placement of a system for gastric electrical stimulation and were compared retrospectively with 18 patients undergoing laparotomy (Open) for identical GES devices for primary diagnosis, past medical history, and health resource utilization from a group of approximately 50 implanted Open patients.4 The GES device was an implantable neuromuscular stimulator (Medtronic Itrel 3) programmed as follows: 330 microseconds, 12-cpm burst frequency, with 5 milliAmperes of current, as previously reported.2,3 This system was approved by the FDA under Humanitarian Device Exemption regulations in 2000, and this study protocol was approved by the University of Arkansas Medical Center Institutional Review Board. Open patients were taken primarily from a group of GES patients who received an implanted investigational device at the University of Tennessee-Memphis, where the device received IRB approval from 1995 to 2000. Written consent forms were obtained from all subjects before the study. The patients at baseline were assessed for a number of variables, including the number of previous abdominal surgeries. After implantation of the GES system (the gastric leads and the pulse generator), symptom improvement was monitored by measurements of Vomiting Frequency (VFS), Nausea Frequency (NFS) scores, and their average (NAVS), (all on a 0 to 4 scale, none to worst); by total gastrointestinal (GI) symptom scores (TSS), the sum of vomiting, nausea, bloating/distension, anorexia/early satiety, and abdominal pain (all scored as 0 to 4, max score 20). Health resource utilization was quantified by an Investigator Derived Independent Outcome Measurement Score (IDIOMS), which quantifies illness severity, health care services, and organ systems involved, (each on a 0 to 10 scale, maximum score 30) as previously reported.4 Gastric emptying was measured by a previously standardized low-fat meal and reported as percentage of solid meal remaining at 4 hours.5 Operative times (OT) in minutes were reported as noted in the patient's operative note, and postoperative hospital length of stay (LOS) in hospital days after surgery were reported. Symptom improvements, health resource measures, measures of gastric emptying (GET), operative times (OT), length of stay (LOS), and long-term survival were reported as mean ± SE and compared by using t tests both within (paired) and between (unpaired) groups.
For both laparoscopy and laparotomy, the surgical goals were similar: gastric serosal electrodes were placed near the body-antral junction, and the device was placed in a subcutaneous pocket. Differences in surgical approach for Lap and Open are noted in the Methods section below.
For Laparoscopy
The patient, after having been anesthetized and draped in a sterile fashion, had an incision made just above the umbilicus. This incision was carried down through the fascia until the peritoneal cavity was entered. A Hasson trocar was placed and pneumoperitoneum was achieved. A 10-mm laparoscopic camera was inserted for intraperitoneal inspection to allow a 10-mm trocar catheter to be placed in the left upper quadrant, and a 5-mm trocar catheter in the right upper quadrant, both under direct vision.
The distal stomach was identified, and the area 8 cm to10 cm proximal to the pylorus was isolated in preparation for the placement of device leads. The stimulation leads, having been prepared outside the body, were then attached to the anterior gastric wall near the greater curvature of the stomach. A sero-muscular partial thickness bite was used to secure the electrodes, so that about 1cm of exposed lead could be attached, leaving the electrode secured in the muscle layers in the wall of the stomach. The lead was then passed through a silastic disk and clipped into place. The disk was tacked in place in 2 positions with suture, and the other end of the lead was also attached to the stomach to prevent migration, using 3– 0 silk. The second lead was placed in an identical manner, about 1 cm from the first lead. Both ends of the leads were then brought out through the 10-mm site in the left upper quadrant. The skin incision was extended, and a subcutaneous pocket was made. Before and after the leads were attached to the pulse generator and then inserted into the pocket, the device was analyzed electronically and when confirmed to be functioning well, the pocket was closed with interrupted 3– 0 Vicryl and then running 4 – 0 Monocryl sutures.
The umbilical incision was closed with 0 Vicryl. The skin was closed at all sites with 4 – 0 Monocryl and sterile dressings were applied. Adequate generator function was confirmed again before the operative procedure was completed.
For Laparotomy
As with laparoscopy, the patient was prepped, draped, and anesthetized, and the abdomen was opened via an upper midline incision with a controlled entrance into the peritoneal cavity. The stomach was identified, inspected, and appropriate sites for electrode placement were identified approximately 8cm proximal to the pylorus. The stimulation leads were secured in a manner similar to that described for laparoscopy above.
After placing the electrodes and securing the leads to the stomach wall, the resistance to electrical stimulation was measured electronically by temporarily attaching the leads to the gastric stimulator, which was placed on the abdominal wall. Gastric electrophysiology was recorded before connecting the leads permanently to the device, to assure that a good quality recording of gastric electrical activity could be obtained from the stimulation leads. As with laparoscopy, a subcutaneous pocket was created lateral to the midline incision, and after passing the leads through the abdominal wall fascia, the leads were once again inserted in the device. An additional measurement of lead impedance was then done before the pocket was closed. The midline surgical wound was then closed in a standard fashion.
RESULTS
Patients were followed up for a mean of 28.8 months in the Lap group and 42.7 months in the Open group. The patients were examined both for their baseline characteristics and for results at the most recent follow-up. Patients with missing data points were included, as long as follow-up was present for at least 6 months.
The 2 patient groups were similar in age (40.8 years for Lap vs 42.0 for Open) and slightly different in sex (6 m 12 f inLapand2m16fin Open). However, fewer Lap patients (6 of 18) had at least 1 previous abdominal surgery than did the Open patients where 11 of 18 had previous abdominal surgery.
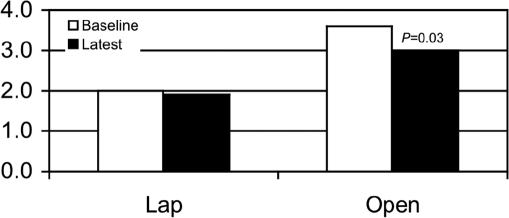
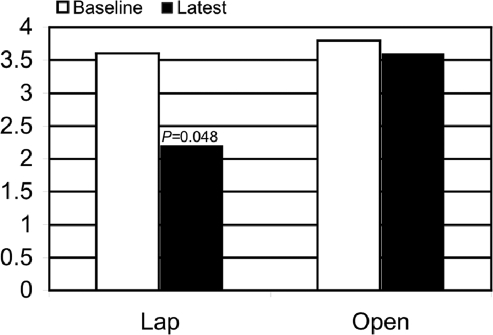
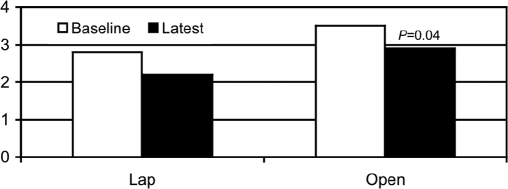
At baseline, the patients with Lap had lower vomiting scores than did the Open group (2.0±0.4 Vomiting Frequency vs 3.6±0.2, Figure 1). The baseline nausea scores were similar 3.6±0.2 Nausea Frequency Score vs 3.8±0.1, (Figure 2) and the baseline average of nausea and vomiting was also less for the Lap patients, but the difference was not as great as baseline vomiting (2.8±0.2 Nausea and Vomiting Score vs 3.5±0.1, Figure 3).
Figure 1.
Vomiting scores. The vomiting score was lower at baseline for the Lap group but improved more at follow-up for the Open group.
Figure 2.
Nausea scores. The nausea scores improved more in the Lap than Open group after gastric stimulation.
Figure 3.
Comparison of combined nausea and vomiting parameters for Lap and Open groups at baseline and following gastric stimulation.
The baseline TSS between the 2 groups was similar (14.3±0.8 vs 16.8±1.2) as were the measures of health resource utilization (17.2±1.0 vs 17.3±1.2).
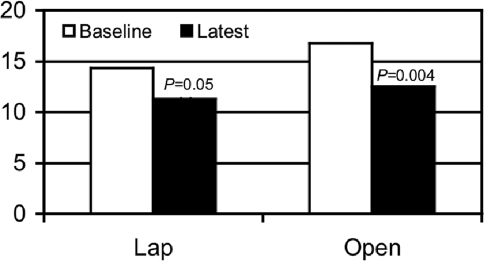
Symptom improvement was similar in both groups, as was improvement in gastric emptying, which is also noted in Tables 1 and 2. At least one of the values of nausea, vomiting, or nausea and vomiting was statistically significant for each group (Table 1). Symptom improvement in the average of nausea and vomiting was from 2.8±0.2 to 2.2±0.3, P=0.1 in the Lap group and from 3.5±0.1 to 2.9±0.3, P<0.05 in the Open group. Symptom improvement in the global measure of TSS was from 14.3±0.8 to 11.3±1.1, P=0.05 in the Lap group and from 16.8±1.2 to 12.5±1.2, P=0.004 in the Open group. (Figure 4)
Table 1.
Summary of Studied Parameters Comparing Lap Versus Open
| Vomiting (V) Baseline | Vomiting (V) Latest | Nausea (N) Baseline | Nausea (N) Latest | N/V Baseline | N/V Latest | TSS Baseline | TSS Latest | |
|---|---|---|---|---|---|---|---|---|
| Lap | 2.0±0.4 | 1.9±0.4 | 3.6±0.3 | 2.2±0.3 | 2.8±0.2 | 2.2±0.3 | 14.3±0.8 | 11.3±1.1 |
| P Value | 0.46 | 0.048 | 0.14 | 0.05 | ||||
| Open | 3.6±0.2 | 3±0.3 | 3.8±0.1 | 3.6±1.7 | 3.5±0.1 | 2.9±0.3 | 16.8 | 12.5 |
| P Value | 0.03 | 0.11 | 0.04 | 0.004 |
Table 2.
Investigator Derived Independent Outcome Measurement Score, Gastric Emptying, Operative Time, and Length of Stay for Lap versus Open
| IDIOMS* Baseline | IDIOMS* Latest | IDIOMS* %change | 4 hr GE* Baseline | 4 Hr GE* Latest | OT* (min) | LOS* (days) | |
|---|---|---|---|---|---|---|---|
| Lap | 17.2±1.0 | 10.2±0.7 | 40 | 20.8±5.2 | 12.4±5.9 | 103.6±8.2 | 1.1±0.1 |
| P Value | <0.001 | ||||||
| Open | 17.3±1.2 | 11.6±0.7 | 30 | 24.1 | 8.8±8.8 | 99.3±10.2 | 6.4±1.4 |
| P Value | <0.001 | ||||||
| Lap/Open P Value | 0.19 | 0.01 |
IDIOMS=Investigator Derived Independent Outcome Measurement Score, GE=gastric emptying, OT=operative time, LOS=length of hospital stay.
Figure 4.
Total symptom score. The total symptoms score was lower at baseline for the Lap group but improved more at follow-up for the Open group.
Between-group improvements in symptoms were also similar: average symptom improvement for Lap was 17.5% vs average symptom improvement of 18% for Open.
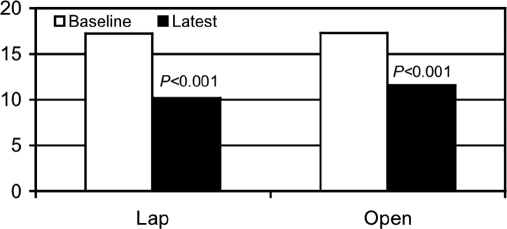
Health resource measures in both groups of patients improved from 17.2±1.0 to 10.2±0.7, P<0.001 in Lap and from 17.3±1.2 to 11.6±0.7, P<0.001 in Open (Figure 5) and were similar as were the between-group comparisons: average change 40% for Lap and 30% for Open.
Figure 5.
Investigator Derived Independent Outcome Measurement Score (IDIOMS). Significant improvement in the Investigator Derived Independent Outcome Measurement Score after gastric stimulation in both groups.
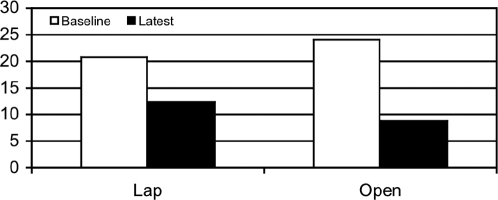
At baseline, the patients with Lap as a group had less retention in gastric emptying than did the Open patients (GET 4 hour % 20.8±5.2 Lap vs 24.1±5.7 for Open) P>0.05. (Figure 6) The percentage of solid meal remaining at 4 hours improved at latest follow-up (12.4±5.9 for Lap and 8.4±8.8 for Open) P>0.05. Examining the patients with delayed GET separately, the majority of patients with delayed GET improved at long-term follow-up and mean values (percentage remaining at 4 hours for all delayed patients in both groups) decreased from 26.0 at baseline to 12.9 at latest follow-up.)
Figure 6.
Gastric emptying. Improvement in gastric emptying in both groups following gastric stimulation but did not reach significant P value.
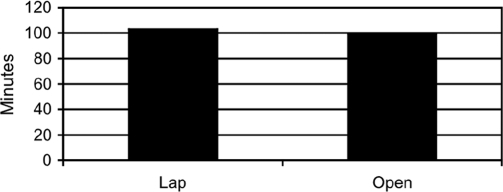
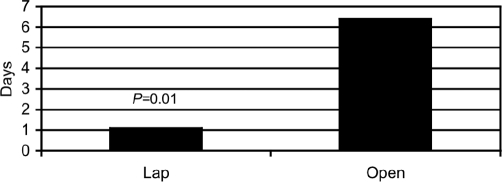
Operative times with Lap were comparable to those for Open (Lap 103.6±8.2 min vs 99.3±10.2 min, P>0.3, Figure 7). However, LOS was significantly shorter with Lap than with Open (1.1±0.1 days, range 0 to 2 days, vs Lot 6.4±1.4 days, range 3 to 14 days, P<0.01, Figure 8). These data are summarized in the accompanying tables, first, for subjective symptom measures and second, for objective health resource, gastric emptying, OT, and LOS data, and the accompanying graphs.
Figure 7.
Comparison of operative times for Open versus Lap groups.
Figure 8.
Length of stay. The length of stay is significantly less for the Lap group.
Over the reported period, 6 patients died, all in the Open group. Five of these deaths were secondary to vascular insults, including MI/CVA, pulmonary embolus, gastrointestinal tract infarction, intracranial bleeding related to anticoagulation therapy, and line sepsis. Three patients had devices replaced during the reported period: 1 in the Lap group (related to an enteral tube replacement) and 2 in the Open group (one from a battery failure, and one due to skin erosion.)
DISCUSSION
Gastric electrical stimulation (GES) based on the technique first applied in 19926,7 has been proven effective for drug-refractory gastroparesis, and was approved as an FDA Humanitarian Use Device (HUD) in 2000 for patients with drug-refractory gastroparesis of idiopathic or diabetic origin. This approval was based on 2 studies, one of which at that point had not been completed, but that showed promising long-term data. The first study (GEMS)2 showed an improvement in nausea and vomiting, using a temporary phase where the device was worn externally, followed by a longer-term study with implanted devices. The results of that second (WAVESS)3 study, a randomized, double-blind, placebo-controlled, cross-over study, have recently been published. The WAVESS study showed a statistically significant reduction in vomiting episodes with the device ON vs OFF. This improvement continued through 1-year follow-up.
An additional study8 has shown short, intermediate, and long-term nutritional benefits of a subset of the GEMS trial followed closely over time. Other controlled trials are underway, and a number of other studies relating to the efficacy of GES in drug-refractory gastroparesis are either underway or the results have been submitted for publication.
Of the 3 electrical stimulation methods advocated for drug-refractory gastroparesis9 (gastric pacing-using high energy and low frequency pulses, gastric electrical stimulation-using low energy and high frequency pulses, and sequential neural stimulation), only the second technique (low-energy, high frequency stimulation) is currently available and was the technique used in this study, as well as the other studies referred to throughout this manuscript.10
To compare the results of laparotomy (Open), the traditional technique used for placement of GES leads and device in patients with drug-refractory gastroparesis, with the results of similar patients who undergo a laparoscopic (Lap) technique, we examined 36 patients, 18 Lap and 18 Open, retrospectively, matched for primary diagnosis and health resource usage. We compared symptoms, gastric emptying, health resource utilization, operative times, and length of postoperative hospital stay, in each of the 2 groups to see what variables, if any, differed at baseline and over time. However, the patients could not be matched for the number of previous abdominal surgeries as mentioned above.
Baseline total gastrointestinal symptoms, gastric emptying, and health resource usage were similar, but the Open patients had higher vomiting and average of nausea and vomiting scores, and had more previous abdominal surgeries. Operative times were similar in the 2 groups, but length of stay was significantly shorter with Lap. Long-term outcome of symptoms, gastric emptying, and health resource utilization were generally comparable, and consistent with that in other published reports.2 However, the Open patients had more deaths during the time reported, suggesting that they were more ill, as a group, than the laparoscopic patients were in this study.
The improvements in gastric emptying times, although worthy of mention, were not statistically significant. This may be due, in part to the fact that at least 4 patients in each group had nondelayed gastric emptying at baseline. Many of the nondelayed patients had postsurgical gastroparesis and in fact had rapid emptying at baseline. It is likely that the inclusion of the values from patients with nondelayed emptying had an impact on the overall GET results at most recent follow-up, as we have shown previously.11
CONCLUSION
We conclude that patients undergoing laparoscopic placement of GES leads and device have shorter lengths of stay, with most other variables being similar. However, the patients who underwent laparotomy had higher vomiting scores at baseline, more previous abdominal surgeries and higher long-term mortality, with most deaths being related to vascular access. Whether earlier (in the course of symptomatic gastroparesis) placement of GES devices would reduce the use of IV access, and possibly mortality, is speculative, and cannot be answered by this report.
We also conclude that laparoscopic placement of GES when feasible, involves shorter hospital stays, with comparable operative times. Improvements in symptom and health resource measures were similar between the 2 groups, as were changes in the gastric emptying times. However the patients in this report undergoing laparotomy may have been more ill at baseline, and had a higher mortality at most recent follow-up. Based on the experience with these gastroparesis patients, laparoscopic placement of GES should be considered whenever technically feasible. Continued improvement in laparoscopic techniques and modifications of equipment for placement GES devices and leads offer the potential for equivalent care with lower hospital costs and equal or improved patient outcomes.
Acknowledgments
The authors would like to thank the study personnel and hospital staffs of the University of Tennessee-Memphis and University of Arkansas for Medical Sciences for help in carrying out the studies on these patients, as well as the Division of Digestive Diseases at University of Mississippi Medical Center for help with manuscript preparation.
Contributor Information
Amar Al-Juburi, Department of Medicine, Division of Gastroenterology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA..
Stephanie Granger, Department of Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA.
James Barnes, Department of Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA.
Guy Voeller, Department of Surgery, UT-Memphis, Memphis, Tennessee, USA.
Derrick Beech, Department of Surgery, UT-Memphis, Memphis, Tennessee, USA.
Hosein Amiri, Department of Surgery, UT-Memphis, Memphis, Tennessee, USA.
Thomas L. Abell, Department of Medicine, Division of Digestive Diseases, University of Mississippi Medical Center, Jackson, Mississippi, USA.
References:
- 1. Luo J, Abell TL, Nash K, Cutts T. Gastric electrical stimulation is associated with reduced health care costs compared with baseline costs and medical controls. Gastroenterology. 1999;116: A77 [Google Scholar]
- 2. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204–212 [DOI] [PubMed] [Google Scholar]
- 3. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428 [DOI] [PubMed] [Google Scholar]
- 4. Cutts TF, Abell TL, Luo J, Eaton P, Kores R. Practitioner-rated diagnostic and predictive score (ADAPS) correlates with quality of life improvement in gastroparesis patients undergoing gastric electrical stimulation. Gastroenterology. 1999;116:A53 [Google Scholar]
- 5. Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462 [DOI] [PubMed] [Google Scholar]
- 6. Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897 [DOI] [PubMed] [Google Scholar]
- 7. Familoni BO, Abell TL, Voeller G, Salem A, Gaber O. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885–891 [DOI] [PubMed] [Google Scholar]
- 8. Abell T, Lou J, Tabbaa M, Batista O, Malinowski S, Al-Juburi A. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. J Parenter Enteral Nutr. 2003;27:277–281 [DOI] [PubMed] [Google Scholar]
- 9. Bortolotti M. The “electrical way” to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883 [DOI] [PubMed] [Google Scholar]
- 10. Hasler WL. Gastric neurostimulation for gastroparesis: Time to pick up the pace. Gastroenterology. 2003;125:979–981 [DOI] [PubMed] [Google Scholar]
- 11. Gaber AO, Oxley D, Karas J, et al. Changes in gastric emptying in recipients of successful combined pancreas-kidney transplants. Dig Dis. 1991;9:437–443 [DOI] [PubMed] [Google Scholar]