Abstract
Objective:
Advanced laparoscopic skills limit the implementation of laparoscopic pyeloplasty to centers with extensive experience. The introduction of robotic technology into the field of minimally invasive surgery has facilitated complex surgical dissection and genitourinary reconstruction. We report our experience with robot-assisted laparoscopic pyeloplasty using the da Vinci Robotic Surgical System at 3 New York City medical centers.
Methods:
A review of all robot-assisted laparoscopic Anderson-Hynes dismembered pyeloplasty cases in 38 patients (21 females, 17 males) between April 2001 and January 2004 was performed. All patients had symptoms or radiographic evidence of ureteropelvic junction obstruction. Robotic assistance with the da Vinci Robotic Surgical System was used after preparation of the ureteropelvic junction with a standard laparoscopic approach.
Results:
The average patient age was 39.3 years (range, 15 to 69). The mean operative time and suturing time were 225.6±59.3 minutes and 64.2±14.6 minutes. The average estimated blood loss was minimal at 77.3±55.3 mL. The mean length of hospitalization was 69.6 hours (range, 28 to 310). The average use of intravenous morphine was 26.5 mg (range, 0 to 162). No intraoperative complications occurred, and open conversions were not necessary. A mean follow-up of 12.2 months revealed a success rate of 94.7% with 2/38 patients requiring further treatments.
Conclusions:
This combined multi-institutional series reveals that robot-assisted pyeloplasty with the da Vinci Surgical System is safe and reproducible. These intermediate results appear comparable to results with open and laparoscopic pyeloplasty repairs.
Keywords: Kidney, Ureteropelvic junction, UPJ, Anderson-Hynes, da Vinci
INTRODUCTION
Open pyeloplasty surgery has traditionally been the standard of care for ureteropelvic junction (UPJ) obstruction in adults, achieving success rates of 90% to 100%.1–3 In an effort to develop a less invasive procedure, percutaneous antegrade and ureteroscopic retrograde endopyelotomy procedures were developed over 20 years ago. Despite lower success rates of 61% to 89% and an increased risk for perioperative hemorrhage,4–9 these endoscopic procedures gained favor for their minimally invasive approaches.
Laparoscopic pyeloplasty was first described in 1993 by Schuessler et al.10 This procedure maintained the benefits of endoscopic approaches, including decreased postoperative pain, short length of hospitalization, and reduced postoperative recovery time, while demonstrating comparable success rates to the conventional open approach.11–14 However, the technical challenge of reconstruction limited this procedure to select medical centers with laparoscopic surgeons with advanced skills.
The introduction of robot-assisted laparoscopic surgery has widened the surgical dimensions for minimally invasive surgery. Specifically, the availability of the da Vinci Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA) has facilitated complex reconstructive and laparoscopic procedures.15–17 The benefits imparted to the surgeon include enhanced 3-D visualization, improved dexterity, greater precision, increased range of motion and reproducibility. We review the initial series of 3 experienced laparoscopic surgeons at several New York City medical centers performing the robot-assisted laparoscopic Anderson-Hynes dismembered pyeloplasty.
METHODS
We performed a review of 38 consecutive patients who underwent robot-assisted laparoscopic pyeloplasty between April 2001 and January 2004 at 3 New York City medical centers. Three physicians (JD, MS, CD) performed all surgery. Flank pain was the presenting complaint for 35/38 patients (92.1%). The other 3/38 patients (7.9%) presented with recurrent pyelonephritis. All 38 patients had symptoms or radiographic confirmation, or both, of ureteropelvic junction obstruction with either a diuretic renal scan or an IVP revealing hydronephrosis and delayed renal function. The 21 female and 17 male patients had disease on the right20 and left.18 All but 2 patients presented with primary ureteropelvic junction obstruction of which 10/38 (26.3%) had crossing vessels identified at the time of surgery. One patient had a renal anomaly of a horseshoe kidney, a second patient had a nonfunctioning contralateral kidney, and 2 more patients had renal calculi associated with the ureteropelvic junction obstruction.
Our technique is similar to other previously described methods.17–20 The da Vinci Robotic Surgical System (Intuitive Surgical, Sunnyvale, CA) available at all 3 of our institutions consists of 2 interactive robotic arms, a camera arm, a 3-dimensional imaging system, and a virtual control chamber. The patient is placed in a lateral or semi-lateral decubitus position and 3 laparoscopic ports [2 8-mm ports (Intuitive Surgical, Sunnyvale, CA),1 12-mm disposable port (Ethicon, Cincinnati, OH)] are placed in a “c” configuration (Figure 1). The ports are placed at least a hand length apart or a minimum of 9 cm away from each other to avoid problems with the working arms of the robot. The beginning portion of the operation is performed as a standard transperitoneal laparoscopic approach. On the left side, the descending colon is displaced medially to gain access to the UPJ. On the right side, the peritoneum is incised from the liver attachments down to the iliac vessels and parallel to the ascending colon, allowing identification of the UPJ between the lower pole of the kidney and the inferior vena cava. The ureter and renal pelvis are also completely mobilized. Extensive dissection of the proximal ureter is avoided to maintain the vascular supply to the ureter and UPJ. Once the diseased UPJ or crossing vessel, or both, is identified, the da Vinci robot is docked into place.
Figure 1.
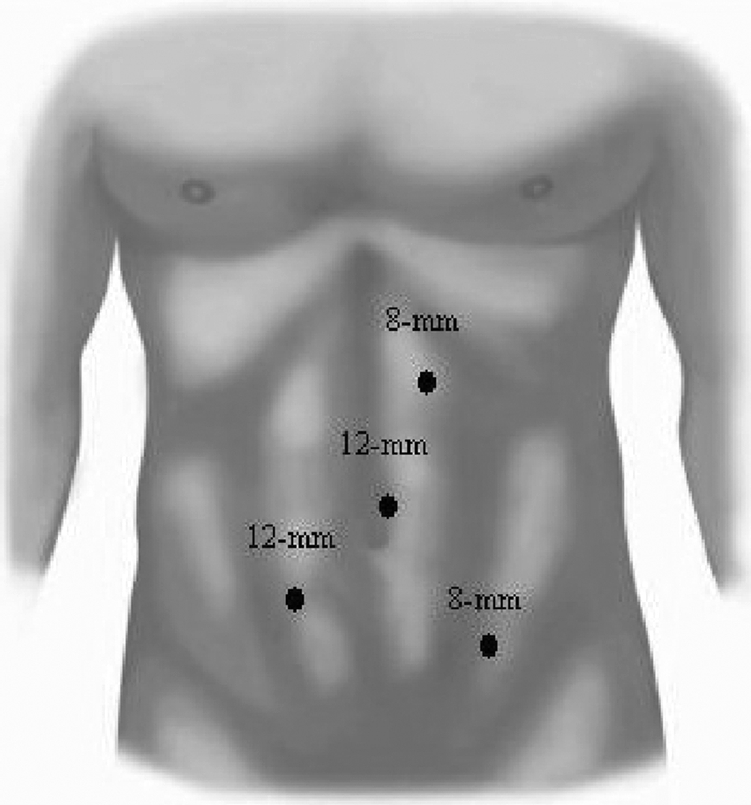
Trocar positioning for left robot-assisted laparoscopic Anderson-Hynes dismembered pyeloplasty. Four laparoscopic ports (two 8-mm ports [Intuitive Surgical, Sunnyvale, CA] and two 12-mm disposable ports [Ethicon, Cincinnati, OH]) are placed in a “c” configuration with 1 contralateral port. The mirror image is done for a right-sided procedure.
Depending on surgeon preference, the camera consisting of a 0° or 30° lens is placed through the disposable 12-mm port at the umbilicus. The robot arms are each placed through the 2 reusable 8-mm ports. At this time, a fourth port or assistant's port is placed in the lower infraumbilical area in a position contralateral to the operative side. One surgeon prefers to place this fourth port at the time of the initial port placements. A 12-mm disposable port allows the assisting surgeon to introduce and retrieve sutures, aid in retraction, and perform suctioning. Robotic instruments used include needle drivers, DeBakey forceps, and Potts scissors.
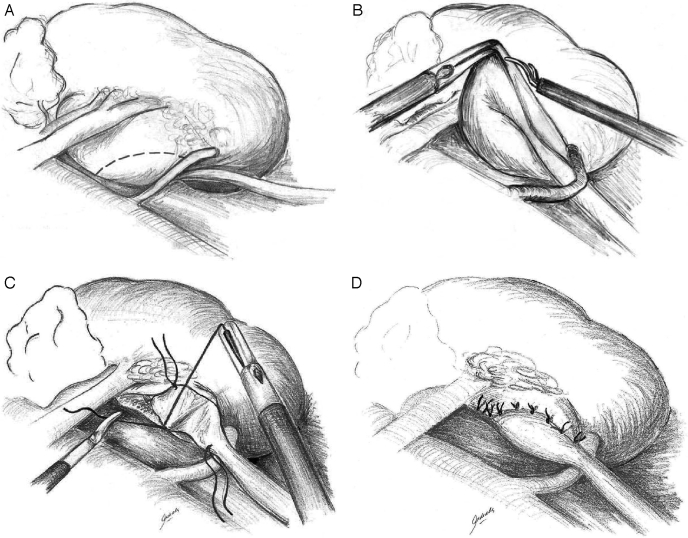
In performing an Anderson-Hynes dismembered pyeloplasty, the renal pelvis is circumferentially transected above the UPJ (Figures 2A and 2B), and the proximal ureter is spatulated laterally. Some earlier cases were performed using laparoscopic endoshears. At this time, the current preference is to spatulate the ureter using the da Vinci Potts scissors. In the case of crossing vessels, the ureter and renal pelvis are transposed to the anterior side of the vessel before initiation of the anastomosis (Figure 2C). If the renal pelvis is redundant, excess tissue is excised. If needed, a concomitant pyelolithotomy was performed before beginning the anastomosis. Stones were either grasped out of the renal pelvis by using a laparoscopic grasper or passing a flexible cystoscope through the assistant's port and then using a stone basket to collect the stones.
Figure 2.
Anderson-Hynes dismembered pyeloplasty is the procedure of choice for robot-assisted cases. (A) dissection of the proximal ureter and ureteropelvic junction reveals a crossing vessel. (B) incision of the renal pelvis and transposition of the crossing vessel. (C) after spatulation of the ureter laterally a posterior anastomosis is performed with running or interrupted sutures. (D) a tension-free, watertight repair is achieved with an internal stent previously placed (not shown).
The anastomosis was begun with an absorbable suture4-0 placed through the apex of the spatulated ureter and at the most dependent portion of the renal pelvis. The posterior anastomosis was then performed with an interrupted or running suture. If redundant pelvis tissue was excised, the remaining pyelotomy incision was closed using additional sutures.
An indwelling ureteral stent is placed in an antegrade fashion over a guidewire and under direct vision. The guidewire is introduced into the abdomen via the assistant's 12-mm port. The wire is then passed down the ureter in an antegrade fashion, and the ureteral stent is passed over the wire. Alternatively, a 16-gauge angiocatheter can also be introduced into the abdomen, allowing a straight access for the wire to pass into the proximal ureter and bladder. The distal coil is positioned within the bladder, and the proximal coil is positioned within the renal pelvis. The Foley catheter is clamped 1 hour earlier to distend the bladder and thus allow the ureteral stent to pass more easily into the bladder. In addition, indigo carmine is instilled into the Foley catheter to observe for backflow into the proximal ureter. With the ureteral stent in place, the anterior anastomosis is then completed (Figure 2D). Following a watertight anastomosis, a drain is placed and then exits the patient via one of the laparoscopic 8-mm ports. In some of our earlier cases, the indwelling stent was placed before surgery, but it is believed that this made the transection and repair of the UPJ more difficult secondary to edema. Correct placement of the distal coil of the ureteral stent into the bladder was also verified with either intraoperative fluoroscopic imaging or a postoperative abdominal x-ray.
The total operative time and suturing time were individually recorded for each patient. The total operative time was based on the operative time from skin to skin. The suturing time was calculated on the actual time that the anastomosis was started and then finished using the da Vinci robot. This time did not include robot set up or docking time. Suturing time included the placement of the ureteral stent placed in an antegrade fashion.
Patients were scheduled for follow-up at 4 weeks to 6 weeks for stent removal. A diuretic renal scan was performed at 3 months and annually thereafter. Clinical follow-up was scheduled annually. Success was defined as improvement of symptoms related to the previous renal obstruction and improved function on diuretic renal scan.
RESULTS
A total of 38 patients (21 females and 17 males) underwent a robot-assisted laparoscopic Anderson-Hynes dismembered pyeloplasty. Average patient age was 39.3 years (range, 15 to 69), and average length of hospitalization was 69.6 hours (range, 28 to 310). Mean operating time was 225.6±59.3 minutes. Mean set up time for the robot was 43±11 minutes. Mean suturing time was 64.2±14.6 minutes. Mean blood loss was 77.3±55.3 mL. The average use of intravenous morphine during hospitalization was 26.5mg (range, 0 to 162). A mean follow-up of 12.2 months revealed that 36/38 (94.7%) patients were unobstructed based on symptoms and radiographic studies.
Obstruction and symptoms persisted in 1 patient after surgery. This patient started with only 15% renal function on the affected side and never recovered function after the robot-assisted pyeloplasty. In addition, the patient continued to have chronic pain on the affected side. Ultimately, a laparoscopic nephrectomy for a nonfunctioning renal unit was performed 3 months later. The patient is now symptom free. A second patient acquired an asymptomatic mild narrowing at the reconstructed anastomosis and was treated with a laser incision of the stricture. Although the patient did not have a documented functional obstruction, it was believed that the patient would develop one in the future. This patient is currently asymptomatic and has a functionally unobstructed renal unit by diuretic renal scan.
Concomitant pyelolithotomy was performed in 2 kidneys that had nonobstructing calculi. All calculi were removed without difficulty. In addition, stones were sent for analysis and were found to be composed of calcium oxalate.
No intraoperative complications occurred, and no open conversions were necessary. Four postoperative complications (10.5%) occurred in our series, 3 of which were minor including 1 patient with a urinary tract infection and 2 patients developing pyelonephritis. The last complication occurred in an early case that involved a 310-pound patient who developed a gluteal compartment syndrome after a prolonged procedure of over 300 minutes.
The time to clear liquids was 17.3 hours (range, 4 to 42) and regular diet was 35.7 hours (range, 20 to 66). The Foley catheter was removed on average 2.4 days after surgery (range, 1 to 5). The JP drain was removed shortly thereafter on average of 2.7 days (range, 1 to 6).
DISCUSSION
Open and endoscopic management of the obstructed UPJ is being challenged by long-term data from laparoscopic pyeloplasty series. Laparoscopy can address both intrinsic and extrinsic causes of obstruction in a manner similar to that of the open approach.10–14
One of the largest published series of 100 laparoscopic pyeloplasty repairs reveals that the authors were able to perform their repairs in an average of 252 minutes. Patients' mean blood loss was 181mL and hospital stay was 3.3 days. Successful outcomes were seen in 96% of their patients with a mean clinical and radiographic imaging follow-up of 2.7 years and 2.2 years.13
According to Jarrett et al,13 the difficulty with the laparoscopic approach is that it is technically challenging and a potentially lengthy surgical procedure due to the high proficiency level required for intracorporeal suturing. With experience, the operative times and learning curve can be reduced to operative times similar to those with open procedures.13
Several experiences with robot-assisted laparoscopic pyeloplasty have demonstrated the feasibility of this technique in providing improved surgical dexterity and decreasing operative times.15–20 Gettman et al17 compared the da Vinci robotic system with standard laparoscopic pyeloplasty. The investigators noted that the Anderson-Hynes pyeloplasty is feasible with either technique. Procedures performed with the da Vinci robot resulted in overall decreased operative times when compared with those of standard laparoscopy. Gettman et al18 also reviewed 9 patients who underwent laparoscopic Anderson-Hynes pyeloplasty with the da Vinci system. The total mean operative time was 138.8 minutes (range, 80 to 215), of which the mean suturing time was 62.4 minutes (range, 40 to 115). Estimated blood loss was less than 50mL in all cases, and the length of hospitalization averaged 4.7 days (range, 4 to 11). Although no intraoperative complications occurred, 1 patient (11.1%) required postoperative open exploration to repair a persistent renal pelvis defect. At short-term follow-up of 4.1 months (range, < 1 to 8 months), all procedures were successful on the basis of the subjective and radiographic data. In this series, robot-assisted laparoscopic pyeloplasty resulted in favorable overall operative times, suturing times, and short-term success rates.
In our series, all 3 surgeons were able to reduce their operative times (skin to skin) from an average of 283.3 minutes for the first 5 cases to 192.0 minutes for the most recent 5 cases (P<0.001). As even more experience is gained with this procedure, it is likely that this operating time can be reduced even further. In addition, all 3 surgeons preferred to start the case with standard laparoscopic techniques. Reasons for this include: 1) Mobilization of the colon, duodenum, liver, spleen, or pancreas is more easily performed with laparoscopic instruments. 2) Being at teaching institutions, the laparoscopic portion allows all members of the surgical team to be involved with the surgery. 3) During the laparoscopic portion, the surgical staff may set up the da Vinci robot.
We exclusively performed the Anderson-Hynes pyeloplasty repair using the da Vinci robot. This repair is the gold standard for open pyeloplasty repairs, and the robot allowed us to duplicate this procedure in the most complicated cases. Intrinsic problems were easily excised and repaired. Extrinsic issues such as crossing vessels were readily addressed.
It is likely that the inexperienced laparoscopic surgeon will gain the most from the da Vinci Surgical System. Besides the experience required to perform complex reconstructive procedures, standard laparoscopic surgery is also handicapped by the reduction in the range of motion due to a fixed trocar position determining the angle of the working field. The robotic instruments are designed with 7 degrees of motion that mimic the dexterity of the human hand and wrist. Each instrument has a specific surgical mission, such as clamping, suturing, and tissue manipulation. In addition, 3-D vision is afforded to the surgeon rather than the 2-dimensional view in standard laparoscopy. Other advantages include potential loss of tremor, decreased trauma to the patient in comparison with trauma in open procedures, and comfort for the surgeon.
Disadvantages include the lack of tactile sensation, and thus visualization of anatomic landmarks is the key to successfully completing the operation. The surgeon is away from the operating table, and therefore must depend on an experienced assistant. Active communication between the primary surgeon, first assistant, and staff is imperative. Although the learning curve for the surgeon may be short (in our experience less than 10 cases), there is a substantial learning curve for the ancillary staff. Many hours of in-servicing may be required, and consistency in the assignment of staff to da Vinci robot cases allows for a smooth transition between cases. Finally, the cost of the da Vinci robot is always a consideration. An initial investment of over $1,000,000 and subsequent running costs of between $80,000 to $100,000 a year, may not make this procedure feasible at many centers. However, as the robotic prostatectomy procedure becomes more popular, the da Vinci Robotic Surgical System may become more readily available at many institutions.
CONCLUSION
This is the largest series of robot-assisted laparoscopic pyeloplasties reported to date. The initial results of this procedure are encouraging, but long-term success rates with follow-up will be needed. The robot-assisted pyeloplasty is safe, reproducible, and feasible between institutions. There is a short learning curve allowing this technique to be easily adopted by motivated surgeons and not just laparoscopically trained surgeons. Our initial results appear comparable to results with open and laparoscopic pyeloplasty repairs. Unfortunately, the cost of acquiring and maintaining a da Vinci robot system may limit the implementation of this procedure at many institutions.
Contributor Information
Michael A. Palese, The Mount Sinai School of Medicine, Department of Urology, New York, New York, USA..
Ravi Munver, Hackensack University Medical Center, Department of Urology, Hackensack, New Jersey, USA..
Courtney K. Phillips, Department of Urology, New York University School of Medicine; New York, New York, USA..
Caner Dinlenc, Department of Urology, Beth Israel Medical Center, New York, New York, USA..
Michael Stifelman, Department of Urology, New York University School of Medicine; New York, New York, USA..
Joseph J. DelPizzo, James Buchanan Brady Foundation, Department of Urology, New York Presbyterian Hospital-Weill Medical College of Cornell University, New York, New York, USA..
References:
- 1. Notley RG, Beaugie JM. The long-term follow-up of Anderson-Hynes pyeloplasty for hydronephrosis. Br J Urol. 1973;45:464–467 [DOI] [PubMed] [Google Scholar]
- 2. Persky L, Krause JR, Boltuch RL. Initial complications and late results in dismembered pyeloplasty. J Urol. 1977;118:162–165 [DOI] [PubMed] [Google Scholar]
- 3. Brooks JD, Kavoussi LR, Preminger GM, Schuessler WW, Moore RG. Comparison of open and endourologic approaches to the obstructed ureteropelvic junction. Urology. 1995;46:791–795 [DOI] [PubMed] [Google Scholar]
- 4. Cassis AN, Brannen GE, Bush WH, Correa RJ, Chambers M. Endopyelotomy: review of results and complications. J Urol. 1991;146:1492–1495 [DOI] [PubMed] [Google Scholar]
- 5. Meretyk I, Meretyk S, Clayman RV. Endopyelotomy: comparison of ureteroscopic retrograde and ante grade percutaneous techniques. J Urol. 1992;148:775–782 [DOI] [PubMed] [Google Scholar]
- 6. Motola JA, Badlani GH, Smith AD. Results of 221 consecutive endopyelotomies: an 8-year follow-up. J Urol. 1993;149:453–456 [DOI] [PubMed] [Google Scholar]
- 7. Nadler RB, Rao GS, Pearle MS, Nakada SY, Clayman RV. Acucise endopyelotomy: assessment of long-term durability. J Urol. 1996;156:1094–1097 [DOI] [PubMed] [Google Scholar]
- 8. Faerber GJ, Richardson TD, Farah N, Ohl DA. Retrograde treatment of ureteropelvic junction obstruction using the ureteral cutting balloon catheter. J Urol. 1997;157:454–458 [PubMed] [Google Scholar]
- 9. Preminger GM, Clayman RV, Nakada SY, et al. A multicenter clinical trial investigating the use of a fluoroscopically controlled cutting balloon catheter for the management of ureteral and ureteropelvic junction obstruction. J Urol. 1997;157:1625–1629 [PubMed] [Google Scholar]
- 10. Schuessler WW, Grune MT, Tecuanhuey LV, Preminger GM. Laparoscopic dismembered pyeloplasty. J Urol. 1993;150:1795–1799 [DOI] [PubMed] [Google Scholar]
- 11. Bauer JJ, Bishoff JT, Moore RG, Chen RN, Iverson AJ, Kavoussi LR. Laparoscopic versus open pyeloplasty: assessment of objective and subjective outcome. J Urol. 1999;162:692–695 [DOI] [PubMed] [Google Scholar]
- 12. Moore RG, Averch TD, Schulam PG, Adams JB, Chen RN, Kavoussi LR. Laparoscopic pyeloplasty: experience with the initial 30 cases. J Urol. 1997;157:459–462 [DOI] [PubMed] [Google Scholar]
- 13. Jarrett TW, Chan DY, Charambura TC, Fugita O, Kavoussi LR. Laparoscopic pyeloplasty: the first 100 cases. J Urol. 2002; 167:1253–1256 [DOI] [PubMed] [Google Scholar]
- 14. Chen RN, Moore RG, Kavoussi LR. Laparoscopic pyeloplasty. Indications, technique, and long-term outcome. Urol Clin North Am. 1998;25:323–330 [DOI] [PubMed] [Google Scholar]
- 15. Sung GT, Gill IS, Hsu TH. Robotic-assisted laparoscopic pyeloplasty: a pilot study. Urology. 1999;53:1099–1103 [DOI] [PubMed] [Google Scholar]
- 16. Sung GT, Gill IS. Robotic laparoscopic surgery: a comparison of the da Vinci and Zeus systems. Urology. 2001;58:893–898 [DOI] [PubMed] [Google Scholar]
- 17. Gettman MT, Peschel R, Neururer R, Bartsch G. A comparison of laparoscopic pyeloplasty performed with the daVinci robotic system versus standard laparoscopic techniques: initial clinical results. Eur Urology. 2002;42:453–457 [DOI] [PubMed] [Google Scholar]
- 18. Gettman MT, Neururer R, Bartsch G, Peschel R. Anderson-Hynes dismembered pyeloplasty performed using the da Vinci robotic system. Urology. 2002;60:509–513 [DOI] [PubMed] [Google Scholar]
- 19. Yohannes P, Burjonrappa SC. Laparoscopic Anderson-Hynes dismembered pyeloplasty using the da Vinci robot:technical considerations. J Endourol. 2003;17:79–83 [DOI] [PubMed] [Google Scholar]
- 20. Bentas W, Wolfram M, Brautigam R, et al. Da Vinci robot assisted Anderson-Hynes dismembered pyeloplasty: technique and 1 year follow up. World J Urol. 2003;21:133–138 [DOI] [PubMed] [Google Scholar]