Abstract
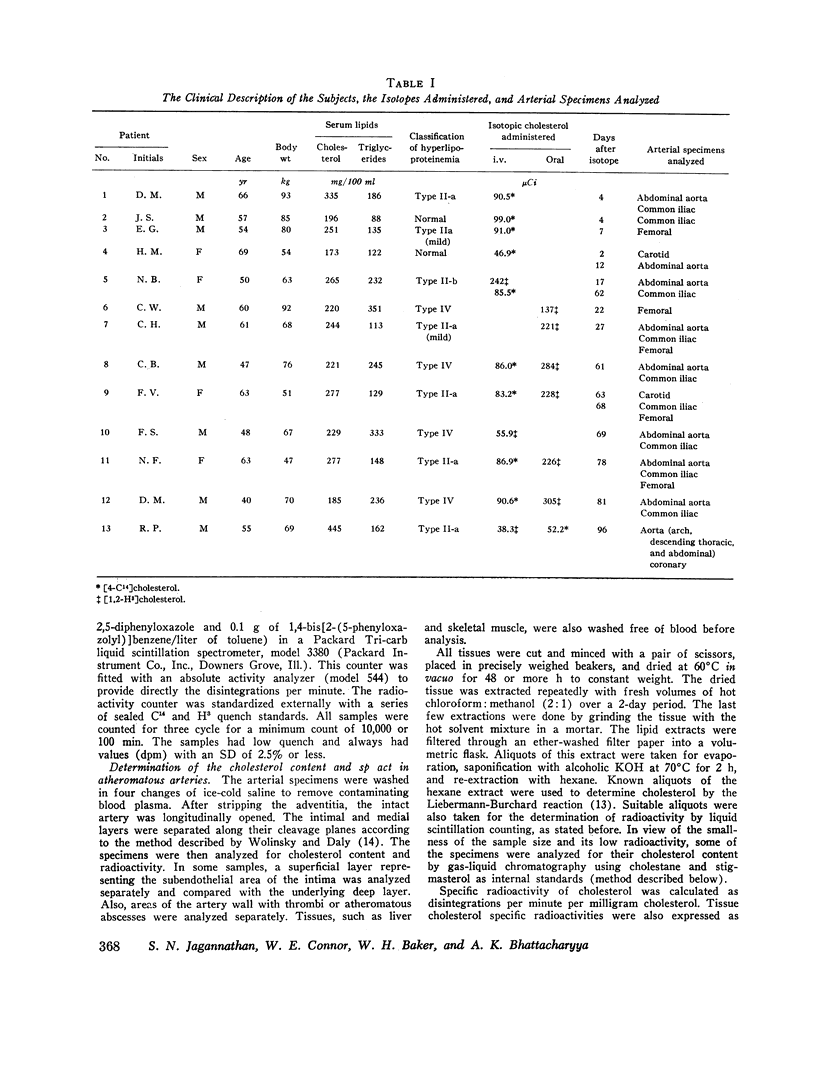
The equilibration of cholesterol between plasma and atherosclerotic arteries was studied in 13 patients with obstructive atherosclerosis 2-96 days after the intravenous and/or oral administration of isotopic cholesterol. Arterial specimens were obtained in 12 patients during surgery for arterial reconstruction and in a 13th patient at autopsy. Equilibration was calculated as the specific radioactivity of cholesterol in the arterial tissue relative to that in the plasma (percent).
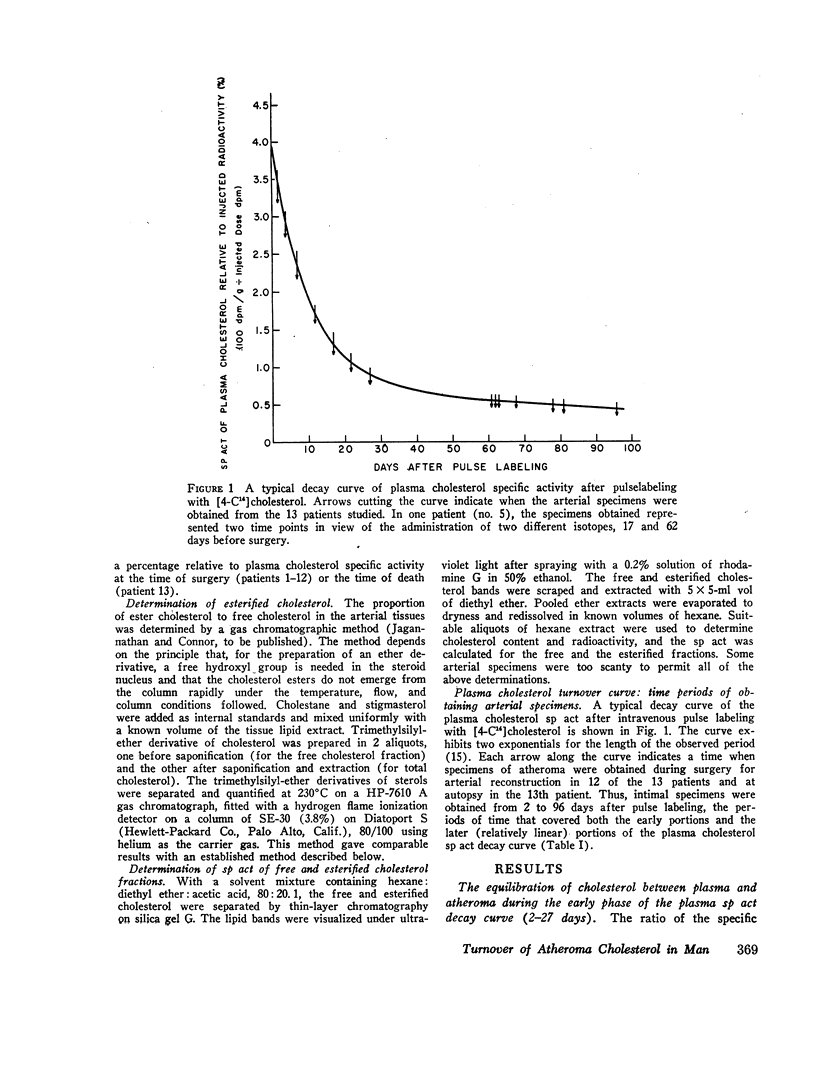
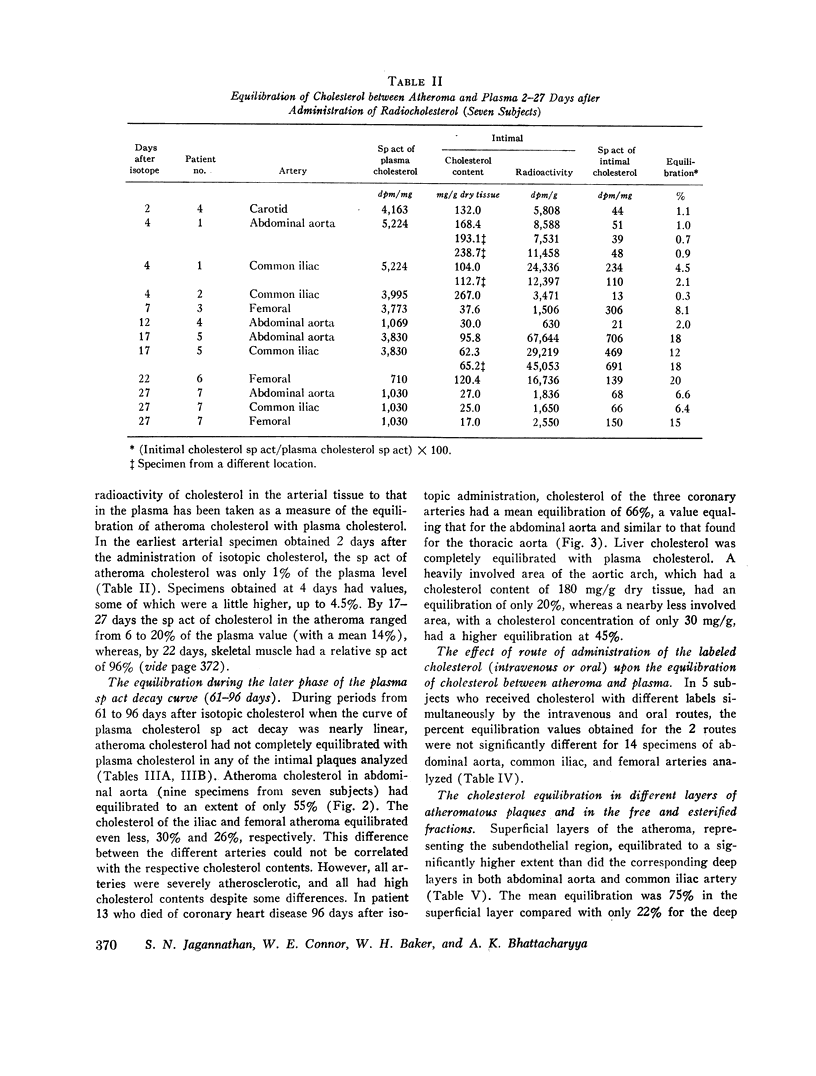
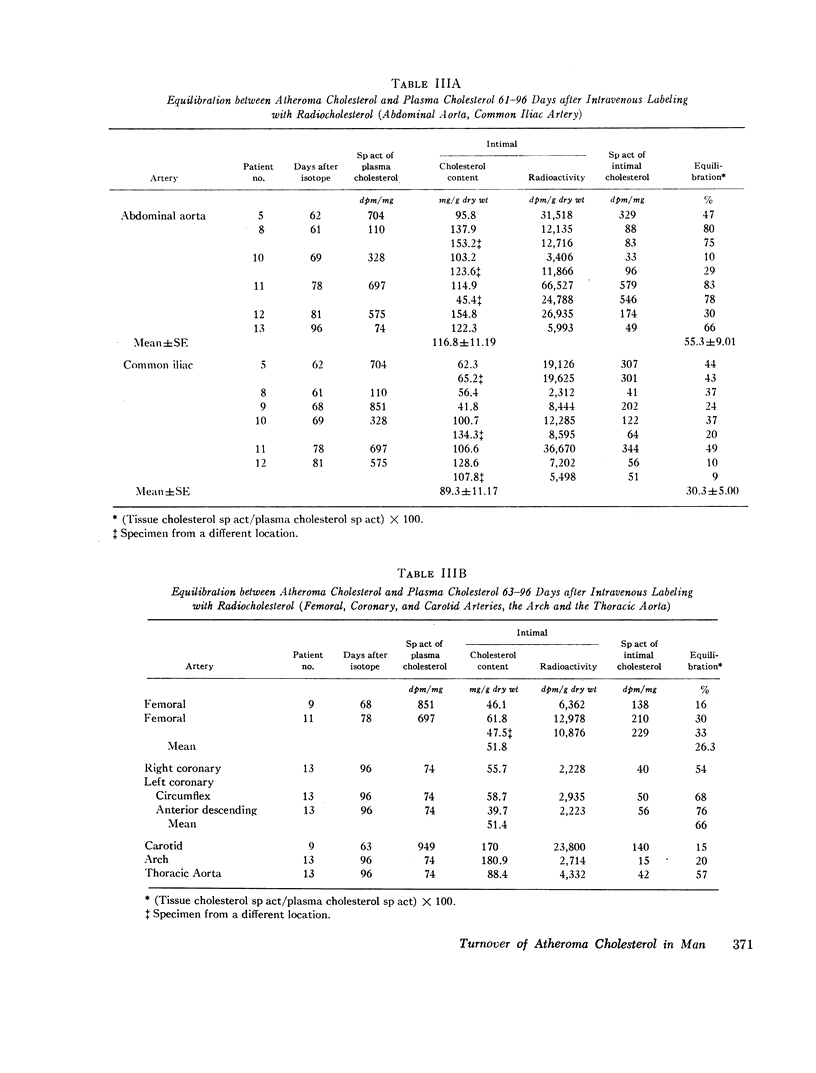
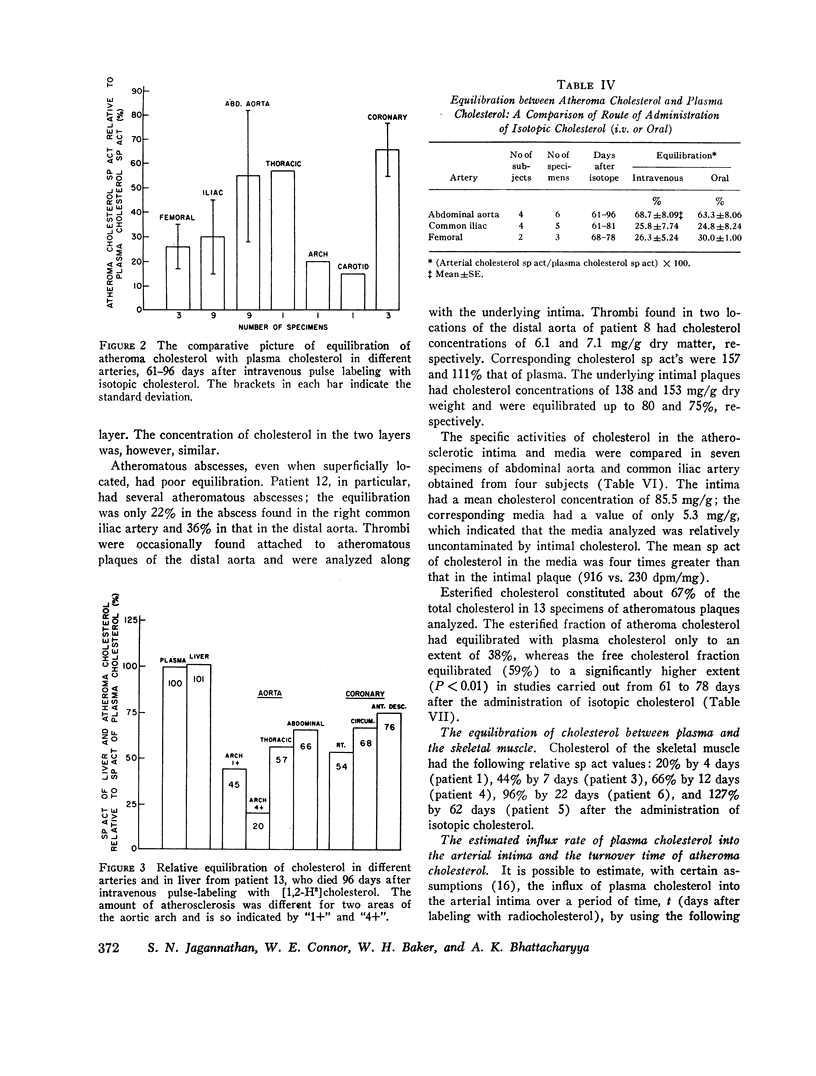
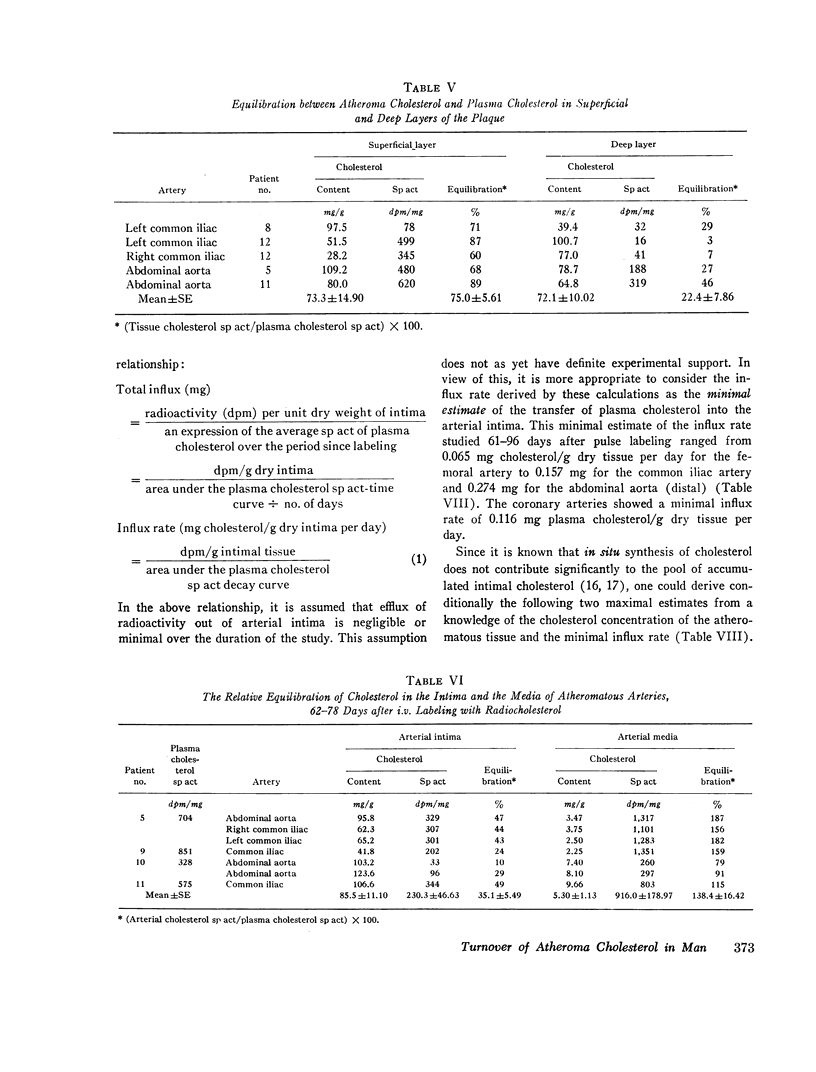
In specimens obtained 2-4 days after pulse labeling, the specific activity of cholesterol in atheroma ranged from 0.3 to 4.5% of that in the plasma. By 17-27 days, the relative specific activity ranged from 6 to 20% in different arteries. In contrast, cholesterol of skeletal muscle had a relative specific activity of 96% by 22 days. By 61-96 days, atheroma cholesterol in the abdominal aorta, common iliac, and femoral arteries had equilibrated to 55, 30, and 26%, respectively. In the patient who died at 96 days, the cholesterol in the coronary arteries had a mean equilibration of 66%, similar to the values for the abdominal (66%) and thoracic (57%) aortas. The route of administration of the isotope did not influence the equilibration.
Within the atheromatous plaque, the superficial layers equilibrated better than the deeper layers (75% vs. 22%). The free cholesterol in the atheroma equilibrated to a significantly higher extent than did esterified cholesterol (59% vs. 38%). There was a fourfold higher specific activity of cholesterol in the media than in the corresponding intima (916 vs. 230 dpm/mg).
The estimated minimal influx rates of plasma cholesterol into the atheromatous intima ranged from 0.065 to 0.274 mg of cholesterol/g dry tissue per day for different arteries. The approximated turnover times of atheroma cholesterol ranged from 442 days for the abdominal aorta and the coronary arteries to 580 days for the common iliac and 821 and 934 days, respectively, for the femoral and the carotid arteries.
These data indicate a definite, though slow, exchange of cholesterol between the plasma and severely atherosclerotic human arteries. Within the atheroma, there are multiple pools of cholesterol, each turning over differently and more slowly than the cholesterol of most other tissues, such as the skeletal muscle. The estimates of influx rate and turnover time of atheroma cholesterol suggest the possibility that this cholesterol is mobilizable, an indication of potential regression of atheromatous lesions in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- AZARNOFF D. L. Species differences in cholesterol biosynthesis by arterial tissue. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):680–683. doi: 10.3181/00379727-98-24150. [DOI] [PubMed] [Google Scholar]
- Armstrong M. L., Megan M. B. Lipid depletion in atheromatous coronary arteries in rhesus monkeys after regression diets. Circ Res. 1972 Jun;30(6):675–680. doi: 10.1161/01.res.30.6.675. [DOI] [PubMed] [Google Scholar]
- Armstrong M. L., Warner E. D., Connor W. E. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970 Jul;27(1):59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- BIGGS M. W., KRITCHEVSKY D., COLMAN D., GOFMAN J. W., JONES H. B., LINDGREN F. T., HYDE G., LYON T. P. Observations on the fate of ingested cholesterol in man. Circulation. 1952 Sep;6(3):359–366. doi: 10.1161/01.cir.6.3.359. [DOI] [PubMed] [Google Scholar]
- BOTTCHER C. J., WOODFORD F. P. Chemical changes in the arterial wall associated with atherosclerosis. Fed Proc. 1962 Jul-Aug;21(4):15–19. [PubMed] [Google Scholar]
- Beaumont J. L., Carlson L. A., Cooper G. R., Fejfar Z., Fredrickson D. S., Strasser T. Classification of hyperlipidaemias and hyperlipoproteinaemias. Bull World Health Organ. 1970;43(6):891–915. [PMC free article] [PubMed] [Google Scholar]
- CHOBANIAN A. V., HOLLANDER W. Body cholesterol metabolism in man. I. The equilibration of serum and tissue cholesterol. J Clin Invest. 1962 Sep;41:1732–1737. doi: 10.1172/JCI104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor W. E., Lin D. S. The intestinal absorption of dietary cholesterol by hypercholesterolemic (type II) and normocholesterolemic humans. J Clin Invest. 1974 Apr;53(4):1062–1070. doi: 10.1172/JCI107643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds L. H., Jr, Fishman N. H., Gregory G. A., Heymann M. A., Hoffman J. I., Robinson S. J., Roe B. B., Rudolph A. M., Stanger P. Cardiac surgery in infants less than six weeks of age. Circulation. 1972 Aug;46(2):250–256. doi: 10.1161/01.cir.46.2.250. [DOI] [PubMed] [Google Scholar]
- FIELD H., Jr, SWELL L., SCHOOLS P. E., Jr, TREADWELL C. R. Dynamic aspects of cholesterol metabolism in different areas of the aorta and other tissues in man and their relationship to atherosclerosis. Circulation. 1960 Oct;22:547–558. doi: 10.1161/01.cir.22.4.547. [DOI] [PubMed] [Google Scholar]
- FRANTZ I. D., Jr, CAREY J. B., Jr, MOSS R., ECKERT J. F., GOLDFARB M., KATZ H. I. Observations on the turnover of cholesterol in a human subject. Minn Med. 1958 Mar;41(3):157–161. [PubMed] [Google Scholar]
- Fredrickson D. S., Levy R. I., Lees R. S. Fat transport in lipoproteins--an integrated approach to mechanisms and disorders. N Engl J Med. 1967 Feb 2;276(5):273–concl. doi: 10.1056/NEJM196702022760507. [DOI] [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Turnover of plasma cholesterol in man. J Clin Invest. 1968 Feb;47(2):231–241. doi: 10.1172/JCI105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND G. S., LUCAS J. L., FREEDBERG A. S. The metabolism of intravenously infused C14-labeled cholesterol in euthyroidism and myxedema. J Lab Clin Med. 1961 Apr;57:574–585. [PubMed] [Google Scholar]
- Lofland H. B., Clarkson T. B. The bi-directional transfer of cholesterol in normal aorta, fatty streaks, and atheromatous plaques. Proc Soc Exp Biol Med. 1970 Jan;133(1):1–8. doi: 10.3181/00379727-133-34394. [DOI] [PubMed] [Google Scholar]
- NEWMAN H. A., ZILVERSMIT D. B. Quantitative aspects of cholesterol flux in rabbit atheromatous lesions. J Biol Chem. 1962 Jul;237:2078–2084. [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Fate of intravenously administered particulate and lipoprotein cholesterol in the rat. J Lipid Res. 1972 Jan;13(1):32–38. [PubMed] [Google Scholar]
- Samuel P., Perl W., Holtzman C. M., Rochman N. D., Lieberman S. Long-term kinetics of serum and xanthoma cholesterol radioactivity in patients with hypercholesterolemia. J Clin Invest. 1972 Feb;51(2):266–278. doi: 10.1172/JCI106811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlqvist M. L., Day A. J., Tume R. K. Incorporation of oleic acid into lipid by foam cells in human atherosclerotic lesions. Circ Res. 1969 Jan;24(1):123–130. doi: 10.1161/01.res.24.1.123. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Daly M. M. A method for the isolation of intima-media samples from arteries. Proc Soc Exp Biol Med. 1970 Nov;135(2):364–368. doi: 10.3181/00379727-135-35052. [DOI] [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]



