Abstract
The prostaglandin (PG) content of mitogen- and antigen-stimulated leukocyte cultures was examined by a radioimmunoassay procedure empolying an antiserum reactive with PGB1 and PGB2, the alkaline dehydration products of PGE and PGA. At 48 h, mitogen-activated mouse spleen cell cultures showed 2-10-fold increases in the PGE, but not in the PGA, component of immunoreactive PG (iPG) fractionated by silicic acid column chromatography. Increases in iPG were detectable by h 16 in spleen cell cultures incubated with staphylococcal enterotoxin B. Since iPG levels rose only in the culture supernates and not in cells exposed to mitogens for 48 h, increases reflected extracellular release of PG. The validity of the radioimmunoassay determinations of PGE in spleen cell cultures was supported by the results of concomitant assessment of the PGE2 content of basal and enterotoxin-stimulated cultures by gas chromatography/mass spectrometry. By the latter method, the PGE2 content was three-fold higher in enterotoxin-activated, compared to basal, cultures at 48 h. Aspirin effectively suppressed increases in both iPG and PGE2. In spleen cell cultures prepared from mice previously inoculated with an attenuated strain of yellow fever virus in vivo and then incubated with this virus in vitro, iPG levels increased twofold over basal at 48 h. By contrast, iPG content of spleen cell cultures prepared from saline-inoculated mice was not appreciably altered by exposure to the virus in vitro.
The enhancement of iPG release from cultured spleen cells by mitogens did not correlate with an ability of these agents to increase cellular cyclic AMP (cAMP) levels. Moreover, epinephrine and cholera toxin markedly increased spleen cell cAMP content but had no demonstrable effect on basal iPG levels, suggesting iPG release from these cells was not mediated by cAMP.
Incubation with mitogens also enhanced the iPG content of 72-cultures of human peripheral leukocytes and of human lymphocytes isolated by nylon chromatography. However, the iPG of cultures of human lymphocytes purified by glass bead chromatography and of mouse thymocytes was not appreciably altered when these cells were cultured with mitogens, even though DNA synthesis in both instances was markedly increased. Accordingly, iPG release was not an invariable concomitant of increased DNA synthesis in lymphoid cell cultures.
In summary, the results demonstrate that mitogen and antigen stimulation of leukocytes in culture may be accompanied by enhanced release of PGE. The mechanisms mediating this phenomenon and its biologic significance remain to be delineated, but participation of PGE in immunologically induced inflammatory responses seems possible.
Full text
PDF

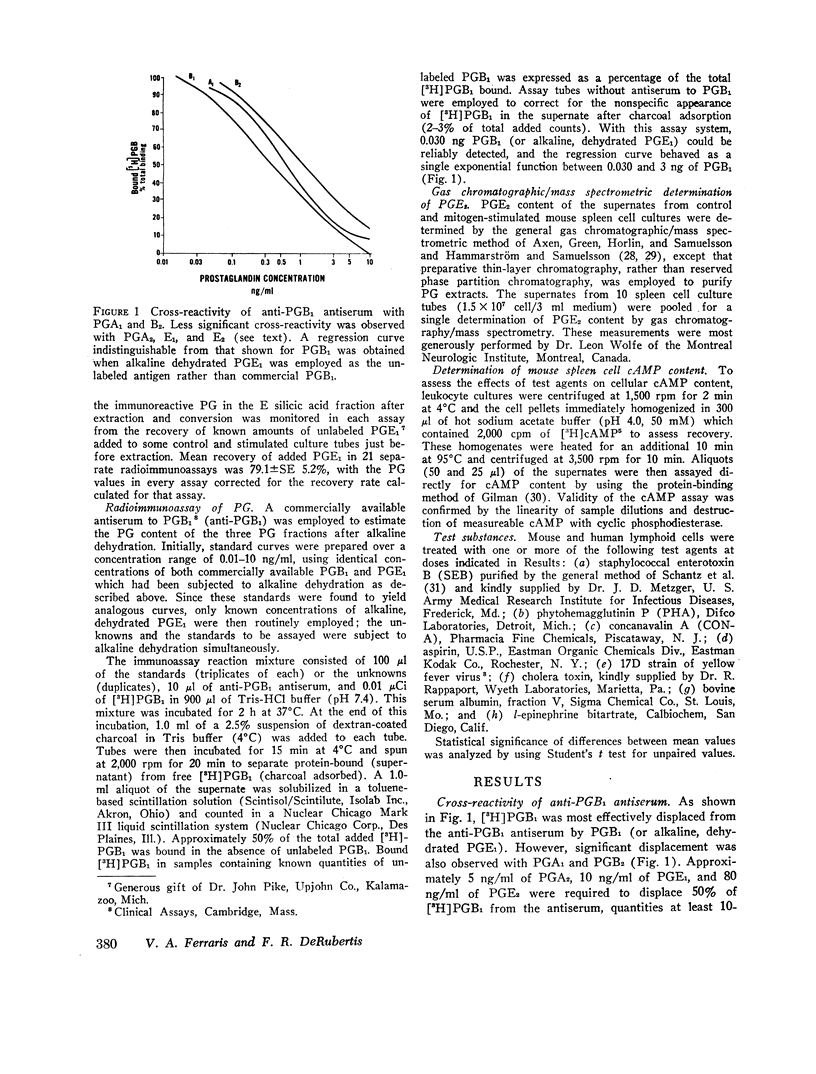
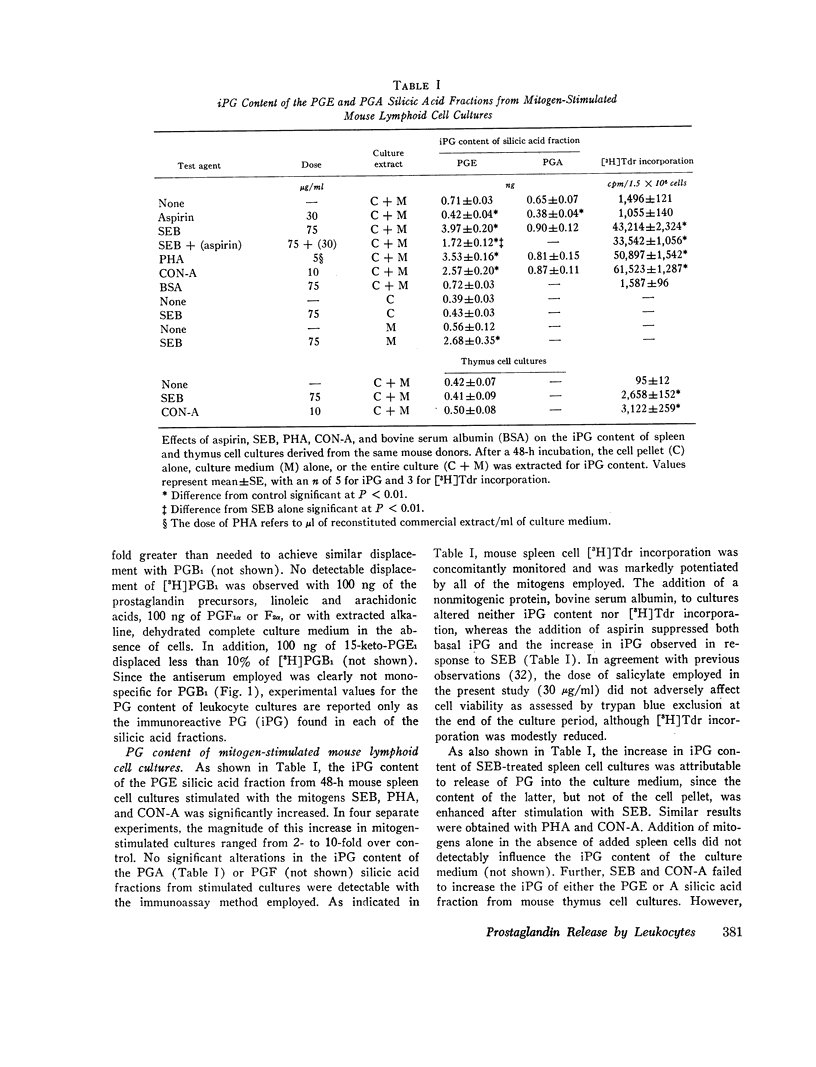
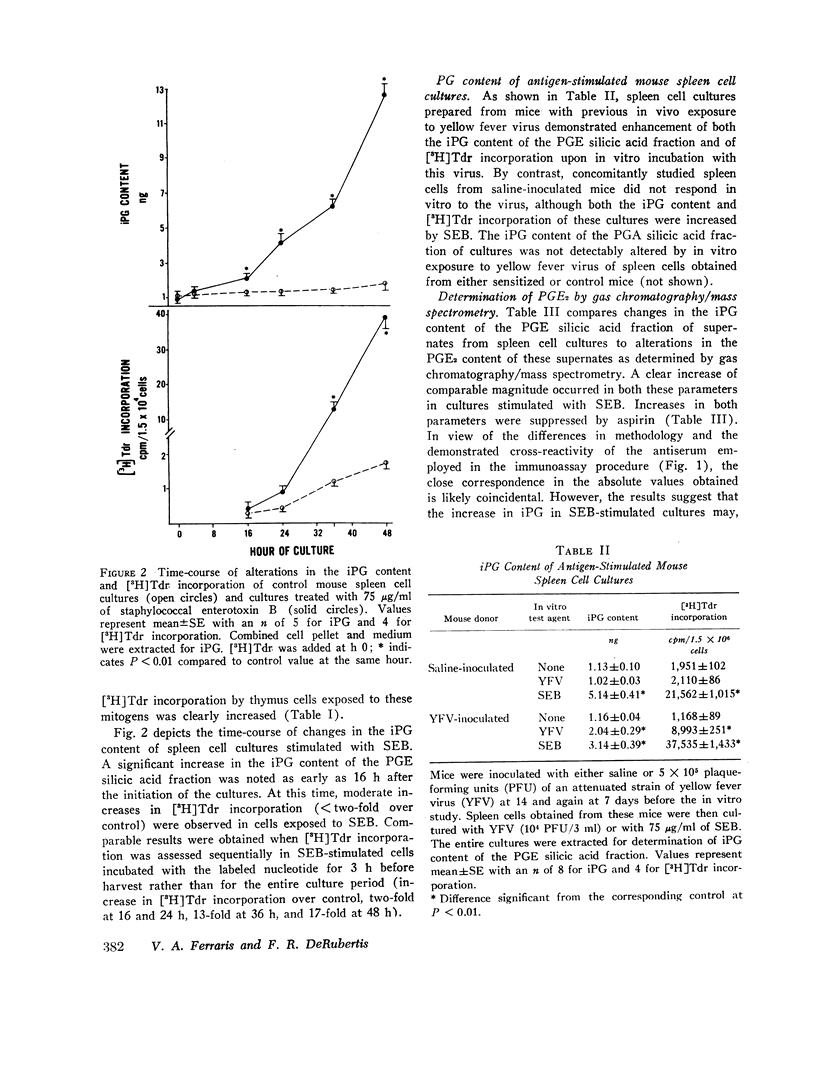
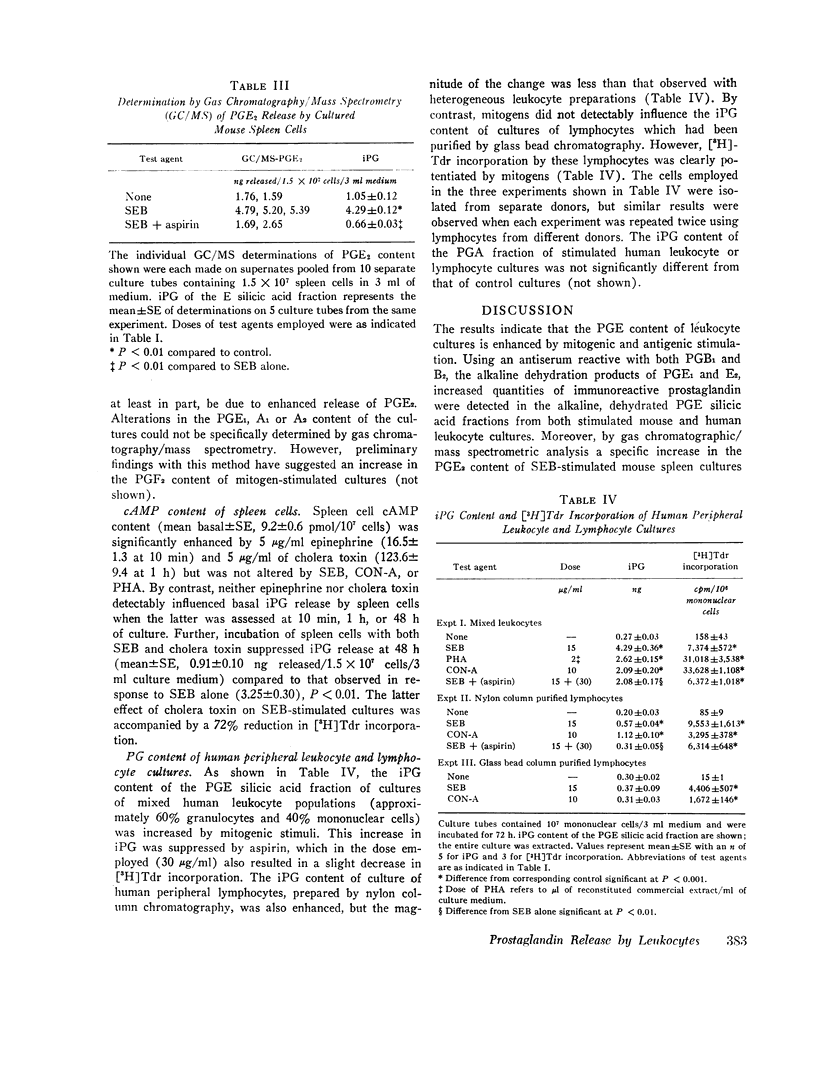



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Adler W. H., Takiguchi T., Marsh B., Smith R. T. Cellular recognition by mouse lymphocytes in vitro. I. Definition of a new technique and results of stimulation by phytohemagglutinin and specific antigens. J Exp Med. 1970 Jun 1;131(6):1049–1078. doi: 10.1084/jem.131.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggård E., Jonsson C. E. Efflux of prostaglandins in lymph from scalded tissue. Acta Physiol Scand. 1971 Apr;81(4):440–447. doi: 10.1111/j.1748-1716.1971.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Axen U., Gréen K., Hörlin D., Samuelsson B. Mass spectrometric determination of picomole amounts of prostaglandins E 2 and F 2 using synthetic deuterium labeled carriers. Biochem Biophys Res Commun. 1971 Oct 15;45(2):519–525. doi: 10.1016/0006-291x(71)90850-3. [DOI] [PubMed] [Google Scholar]
- Bennett A., Fleshler B. Prostaglandins and the gastrointestinal tract. Gastroenterology. 1970 Nov;59(5):790–800. [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L. Pharmacologic control of allergic histamine release in vitro: evidence for an inhibitory role of 3',5'-adenosine monophosphate in human leukocytes. J Immunol. 1972 Mar;108(3):695–705. [PubMed] [Google Scholar]
- Burke G. Effects of thyrotropin and N6,02'-dibutyryl cyclic 3'.5'-adenosine monophosphate on prostaglandin levels in thyroid. Prostaglandins. 1973 Mar;3(3):291–297. doi: 10.1016/0090-6980(73)90067-1. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Swett V. C. The interaction of human monocytes and lymphocytes. J Exp Med. 1968 Dec 1;128(6):1309–1325. doi: 10.1084/jem.128.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunkhorn P., Willis A. L. Cutaneous reactions to intradermal prostaglandins. Br J Pharmacol. 1971 Jan;41(1):49–56. doi: 10.1111/j.1476-5381.1971.tb09934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber E., Resch K. Phospholipid metabolism of stimulated lymphocytes: activation of acyl-CoA:lysolecithin acyltransferases in microsomal membranes. Biochim Biophys Acta. 1973 Feb 14;296(2):335–349. doi: 10.1016/0005-2760(73)90092-1. [DOI] [PubMed] [Google Scholar]
- Finck A. D., Katz R. L. Prevention of cholera-induced intestinal secretion in the cat by aspirin. Nature. 1972 Aug 4;238(5362):273–274. doi: 10.1038/238273a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. W., Sondergaard J., McDonald-Gibson W. Recovery of prostaglandins in human cutaneous inflammation. Br Med J. 1971 May 1;2(5756):258–260. doi: 10.1136/bmj.2.5756.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Samuelsson B., Bjursell G. Prostaglandin levels in normal and transformed baby-hamster-kidney fibroblasts. Nat New Biol. 1973 May 9;243(123):50–51. [PubMed] [Google Scholar]
- Henney C. S., Bourne H. R., Lichtenstein L. M. The role of cyclic 3',5' adenosine monophosphate in the specific cytolytic activity of lymphocytes. J Immunol. 1972 Jun;108(6):1526–1534. [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Horton J. E., Raisz L. G., Simmons H. A., Oppenheim J. J., Mergenhagen S. E. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science. 1972 Sep 1;177(4051):793–795. doi: 10.1126/science.177.4051.793. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Behrman H. R., Parker C. W. Radioimmunoassay measurement of prostaglandins E, A, and F in human plasma. J Clin Invest. 1973 Feb;52(2):398–405. doi: 10.1172/JCI107196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaley G., Weiner R. Prostaglandin E-1: a potential mediator of the inflammatory response. Ann N Y Acad Sci. 1971 Apr 30;180:338–350. doi: 10.1111/j.1749-6632.1971.tb53203.x. [DOI] [PubMed] [Google Scholar]
- Klein D. C., Raisz L. G. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970 Jun;86(6):1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- Levine L., Hinkle P. M., Voelkel E. F., Tashjian A. H., Jr Prostaglandin production by mouse fibrosarcoma cells in culture: inhibition by indomethacin and aspirin. Biochem Biophys Res Commun. 1972 May 26;47(4):888–896. doi: 10.1016/0006-291x(72)90576-1. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L. M., Henney C. S., Bourne H. R., Greenough W. B., 3rd Effects of cholera toxin on in vitro models of immediate and delayed hypersensitivity. Further evidence for the role of cyclic adenosine 3',5'-monophosphate. J Clin Invest. 1973 Mar;52(3):691–697. doi: 10.1172/JCI107230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Prostaglandins: mediators of inflammation? Science. 1972 Sep 1;177(4051):780–781. doi: 10.1126/science.177.4051.780. [DOI] [PubMed] [Google Scholar]
- Pachman L. M., Esterly N. B., Peterson R. D. The effect of salicylate on the metabolism of normal and stimulated human lymphocytes in vitro. J Clin Invest. 1971 Jan;50(1):226–230. doi: 10.1172/JCI106478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper P., Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci. 1971 Apr 30;180:363–385. doi: 10.1111/j.1749-6632.1971.tb53205.x. [DOI] [PubMed] [Google Scholar]
- Ramwell P. W., Shaw J. E. Biological significance of the prostaglandins. Recent Prog Horm Res. 1970;26:139–187. doi: 10.1016/b978-0-12-571126-5.50008-x. [DOI] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- Shio H., Shaw J., Ramwell P. Relation of cyclic AMP to the release and actions of prostaglandins. Ann N Y Acad Sci. 1971 Dec 30;185:327–335. doi: 10.1111/j.1749-6632.1971.tb45257.x. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Newberry W. M., Jr, Parker C. W. Cyclic adenosine 3',5'-monophosphate in human lymphocytes. Alterations after phytohemagglutinin stimulation. J Clin Invest. 1971 Feb;50(2):432–441. doi: 10.1172/JCI106510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L. M., Juhlin L., Kirschenbaum M. B. Prostaglandin on cutaneous vasculature. J Invest Dermatol. 1968 Oct;51(4):280–282. [PubMed] [Google Scholar]
- Sondergaard J., Greaves M. W. Recovery of a pharmacologically active fatty acid during the inflammatory reaction, invoked by patch testing in allergic contact dermatitis. Int Arch Allergy Appl Immunol. 1970;39(1):56–61. doi: 10.1159/000230333. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Moddox I. S. Prostaglandin production by experimental tumours and effects of anti-inflammatory compounds. Nat New Biol. 1972 May 10;237(71):59–61. doi: 10.1038/newbio237059a0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L., Goldhaber P. Evidence that the bone resorption-stimulating factor produced by mouse fibrosarcoma cells is prostaglandin E 2 . A new model for the hypercalcemia of cancer. J Exp Med. 1972 Dec 1;136(6):1329–1343. doi: 10.1084/jem.136.6.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Adv Immunol. 1972;15:95–165. doi: 10.1016/s0065-2776(08)60684-7. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Weiner R., Kaley G. Influence of prostaglandin E1 on the terminal vascular bed. Am J Physiol. 1969 Aug;217(2):563–566. doi: 10.1152/ajplegacy.1969.217.2.563. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A. Effects of prostaglandins PGF2a and PGE1 on vascular permeability. J Pathol Bacteriol. 1968 Oct;96(2):381–387. doi: 10.1002/path.1700960216. [DOI] [PubMed] [Google Scholar]



