Abstract
Objective:
Gastrointestinal stromal tumors are the most common submucosal masses in the stomach and are mostly benign. Minimally invasive surgery is being increasingly used for their excision. Tumors close to the cardia often require a stapled resection of stomach. We report a technique for enucleating a 4-cm, well-circumscribed gastric submucosal tumor at the cardia, avoiding gastric transection.
Methods:
A gastroscope was introduced to distend the stomach. A laparoscope was inserted through the umbilicus after pneumoperitoneum was created. Two 5-mm metal trocars were inserted into the stomach under vision. A 10-mm trocar was passed through the umbilical incision into the stomach for the camera. Dilute epinephrine was injected submucosally. The tumor was enucleated after incising the overlying mucosa. A gastroscope snare helped in grasping the tumor for retraction and final removal in a plastic bag. The submucosal defect and gastric port-site defects were sutured laparoscopically with Vicryl 3– 0.
Results:
Contrast studies showed no leakage. Final histology indicated a benign leiomyoma. The patient was discharged on the sixth day. No recurrence was noted at 6-month follow-up.
Conclusions:
Benign stromal tumors at the cardia can be safely enucleated by this method. This technique is cost-effective as it avoids expensive staplers or self-retaining gastric balloon ports.
Keywords: Laparoendoscopic intragastric enucleation, Gastrointestinal stromal tumor (GIST), Cardia
INTRODUCTION
Gastrointestinal stromal tumors (GIST) account for 1% to 3% of all resected gastric tumors. They are mostly benign and are the commonest submucosal mass in the stomach.1 The preoperative characterization of malignancy is often difficult, and excision is the most common management option.2 The deformity after resection of a lesion at the cardia can result in gastroesophageal reflux or late stenosis.3 We describe herein a technique to enucleate a 4-cm well-circumscribed, benign submucosal tumor at the cardia, avoiding the need for gastric resection.
CASE REPORT
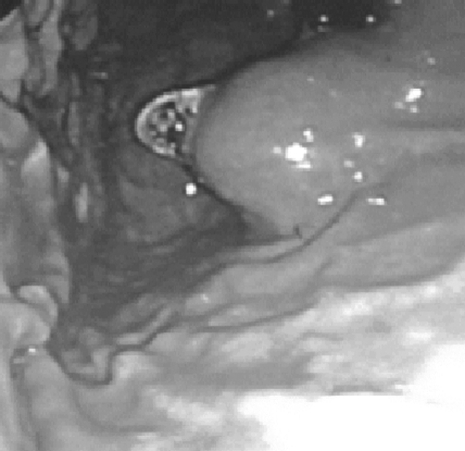
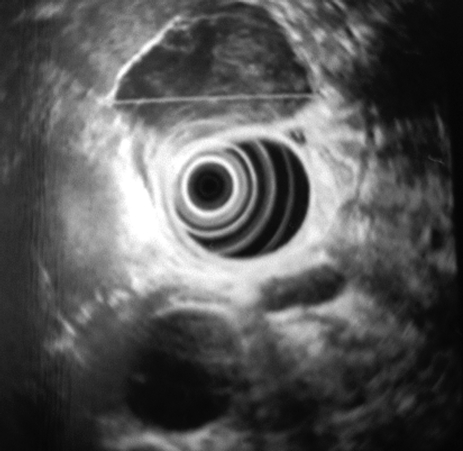
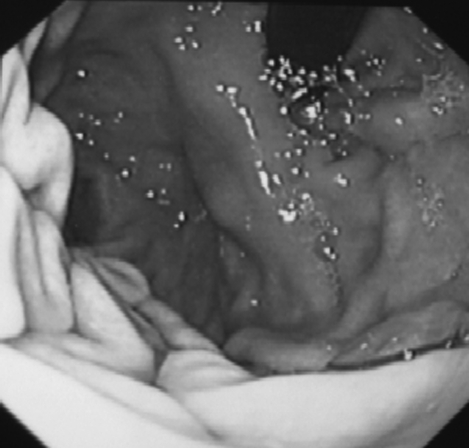
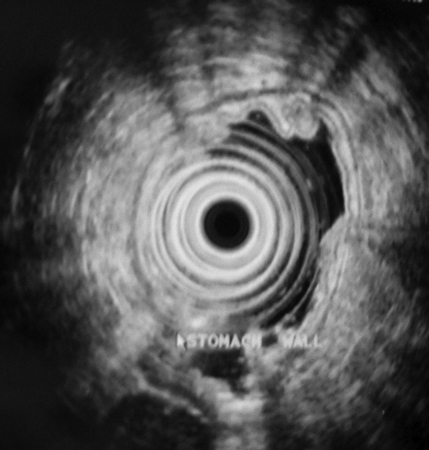
A 52-year-old previously healthy male presented with symptoms of epigastric pain and dyspepsia. Physical examination and blood investigations were normal. Gastroscopy revealed antral gastritis and a smooth, well-defined, 4-cm mass lesion at the cardia. The overlying mucosa was normal (Figure 1). A subsequent endoscopic ultrasound (EUS) confirmed the lesion to be well-circumscribed and submucosal in location (Figure 2).
Figure 1.
Gastroscopy shows a stromal tumor at the cardia with normal mucosa.
Figure 2.
Endoscopic ultrasound (EUS) showing a 4-cm, well-circumscribed submucosal tumor.
After treatment of the gastritis, a decision to proceed with a combined laparoendoscopic enucleation of the tumor was made.
Operative Technique
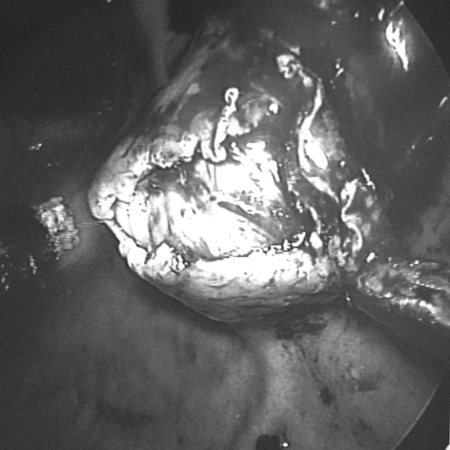
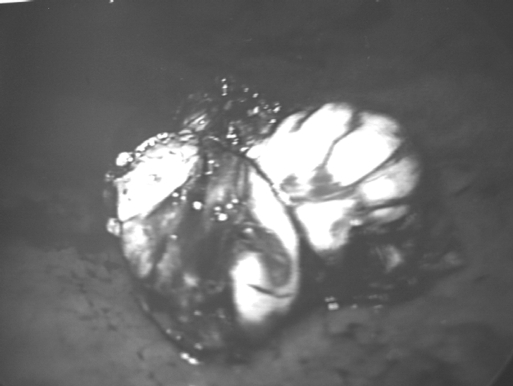
The patient was placed in a modified lithotomy position after being put under general anesthesia. The surgeon stood on the right side and operative assistant on the left. The laparoscope assistant stood between the patient's legs. An initial diagnostic laparoscopy was performed after an open pneumoperitoneum was created through the umbilicus. A gastroscope was inserted to inflate the stomach. Two 5-mm metal trocars were inserted transabdominally into the stomach under view of the laparoscope and gastroscope. The 10-mm umbilical port was then advanced into the gastric lumen to allow the camera to pass in and visualize the tumor. The presence of the gastroscope allowed us to visualize the safe entry of all the ports into the stomach. Anchoring skin stitches around the ports secured them in place and prevented inadvertent slippage from the stomach. Dilute epinephrine (1:5 x 106) was injected into the submucosal plane beneath the tumor to reduce bleeding and facilitate dissection. The mucosa over the tumor was incised with a diathermy hook (Figure 3), and carefully peeling away the overlying mucosa and underlying muscle separated the tumor. An endoscopic snare through the gastroscope was used to grasp the tumor (Figure 4) and aid in retraction and final removal through the oral cavity in a plastic bag. The gastric submucosal defect (Figure 5) was closed intraluminally by 3– 0 Vicryl sutures. After hemostasis, a nasogastric tube was placed in the stomach, and the 3 ports were withdrawn from the stomach. The gastric port-site defects were also closed laparoscopically with continuous Vicryl 3– 0 sutures. The stomach showed no leakage intraoperatively when tested with colored dye.
Figure 3.
Mucosa incised over the tumor.
Figure 4.
Tumor grasped by endoscopic snare.
Figure 5.
Submucosal defect after excision of tumor.
RESULTS
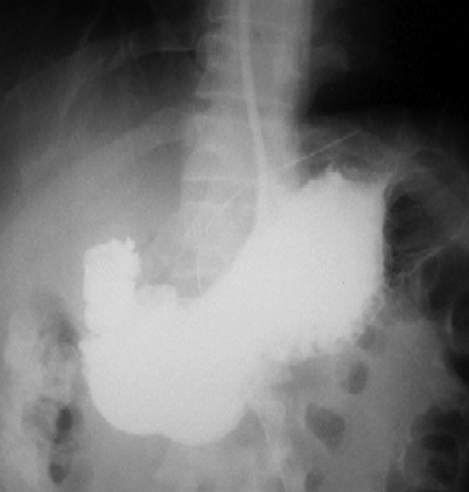
An oral Gastrografin study on the fifth postoperative day demonstrated no leakage (Figure 6). The patient had an uneventful recovery and was discharged on the sixth day. The final histology indicated a benign leiomyoma. Follow-up gastroscopy (Figure 7) and EUS (Figure 8) 6 months later showed no recurrence. The patient had no dysphagia or symptoms of gastroesophageal reflux.
Figure 6.
Contrast study on fifth day showed no leak.
Figure 7.
Gastroscopy at 6 months showed no recurrence.
Figure 8.
Endoscopic ultrasound (EUS) at 6 months showed no residual tumor or recurrence.
DISCUSSION
Gastrointestinal stromal tumor (GIST) is the preferred term for leiomyomas, leiomyoblastomas, and leiomyosarcomas because they have been shown to originate from undifferentiated stromal fibroblasts.1 They are the commonest submucosal masses found in the stomach, comprising 1% to 3% of all resected gastric neoplasms. The majority of these are benign and asymptomatic.2–5 They may occasionally present as symptoms of obstruction, compression or hemorrhage.6,7 Hemorrhage is the most common symptom of malignancy.6 Malignant lesions account for 20% of all GISTs.6 Malignancy is indicated only by metastasis or local invasion noted at the time of surgery or after resection.2,3 Histological diagnosis from biopsy specimens is difficult.4 For further definition, endoscopic ultrasound (EUS) and ultrasound-guided needle aspiration have been used. EUS characterizes the muscular origin of these lesions in the gastric wall and the well-defined margins of benign tumors. Thus in the majority of cases, the diagnosis is established and FNAC is not warranted.6 In our patient, EUS (Figure 2) showed the tumor to be well circumscribed and arising from the lamina muscularis with sparing of the muscularis propria. This finding on EUS is classical of a benign stromal tumor. Histological diagnosis from biopsy specimens is difficult for these submucosal lesions and therefore no biopsies were taken during the gastroscopy.
Walsh and Heniford6 suggest that once diagnosed, a GIST of any size should be excised to eliminate the need for serial endoscopy and prevent the progression of symptoms. Moreover, a size greater than 6cm is more commonly associated with malignancy. Cheng et al,2 however, believe that surgical intervention is not required for small, clinically insignificant lesions and that resection is indicated only for large or symptomatic lesions.
The recommended methods for excision of GIST tumors include local excision with a 2-cm to 4-cm margin,2,3 enucleation8 and stapled gastric resection.5,9 The latter option is especially indicated if evidence of malignancy is present.8,10 All these procedures can be performed by laparoscopic or open methods.
Minimally invasive methods described in the literature range from a purely endoscopic technique,11,12 to a combined endolaparoscopic6,7,13,14 to a purely laparoscopic method.4,5,8 The technique can be performed intracorporeally or extracorporeally,15 intragastrically8,14 or transgastrically.4,5 Position is an important factor in determining the approach used. Anterior wall lesions and those situated along the curvatures generally undergo wedge resection. Posterior wall lesions undergo a gastrostomy and resection, whether intragastric or transgastric. For tumors located at the cardia, laparoscopic gastric resection using an exogastric or intragastric approach is difficult.9 Due to its location, resection with sufficient margins is not possible without compromising the Angle of His and the muscle layers that construct the antireflux mechanism.14 The deformity after resection of a lesion in the gastric fundus and cardia can result in gastroesophageal reflux or stenosis.16
According to Taniguchi et al,8 enucleation is a satisfactory procedure for benign gastric leiomyomas. However, for gastric leiomyosarcomas, a wider resection of the full thickness of the gastric wall with adequate margins is necessary. As partial resection of the stomach with adequate margins cannot be applied to tumors close to the cardia, they have been subjected to major surgery, such as total or proximal gastrectomy.8 Such major surgery would be excessive if the tumor is proven to be benign at postoperative pathological examination. Hence, they recommend that for GIST tumors at the cardia that look benign and have low malignant potential, enucleation is a better option. If the tumor were proven to be benign, as suspected preoperatively, the enucleation would be sufficient and give the patient a less-invasive outcome. The potential problems of reflux and stenosis are also avoided. If, however, the enucleated tumor is diagnosed as malignant, a second-look operation will be necessary, and based on the full pathological information the optimum gastric resection performed. Concerning the potential for tumor seedings, it is believed that the risks are negligible because almost all the tumors to which this technique is applied would be low-grade malignancies that appear benign.
In our patient, the endoscopic ultrasound features were quite classical of a benign stromal tumor. We therefore proceeded to perform a combined laparoendoscopic intragastric enucleation. Since the tumor was only 4 cm and could be retrieved orally, only 3 openings in the stomach for the trocars were needed. Furthermore, under the magnified view of the laparoscope, the relationship between the tumor and the Z line or the gastric muscle layers could be clearly seen. This facilitated the preservation of the antireflux mechanism and avoided stenosis. Follow-up gastroscopy and EUS is recommended for early detection of recurrence.
This technique can also be applied to lesions situated on the posterior gastric wall. However, it is not suitable for tumors on the anterior wall or those showing extragastric growths. An EUS is mandatory to confirm the origin, and in suspicious cases a guided biopsy may be advisable to confirm the benign nature of the lesion before proceeding with this method.
CONCLUSION
Although technically demanding, this is a safe, feasible technique for removal of well-circumscribed benign stromal tumors at the cardia. By avoiding a gastric resection in this region, the risks of gastroesophageal reflux or stenosis can be prevented.
References:
- 1. Bennet MK. Pathology of esophageal and gastric cancers. In: Griffin SM, Raimes SA. eds. Upper gastrointestinal surgery. London: WB Saunders; 1997; 1–35 [Google Scholar]
- 2. Cheng HL, Lee Wj, Lai IR, Yuan RH, Yu SC. Laparoscopic wedge resection of benign gastric tumour. Hepatogastroenterology. 1999; 46: 2100–2104 [PubMed] [Google Scholar]
- 3. Llorente J. Laparoscopic gastric resection for gastric leiomyomas. Surg Endosc. 1994; 8: 887–889 [DOI] [PubMed] [Google Scholar]
- 4. Rohatgi A, Singh KK. Laparoendoscopic management of gastrointestinal stromal tumors. J Laparoendosc Adv Surg Tech A. 2003; 13 (1): 37–40 [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim IM, Silvestri F, Zingler B. Laparoscopic resection of a posterior gastric leiomyoma using combined and gastroscopic approach. Surg Endosc. 1997; 11: 277–279 [DOI] [PubMed] [Google Scholar]
- 6. Walsh RM, Heniford BT. Laparoendoscopic treatment of gastric stromal tumors. Semin Laparosc Surg. 2001; 8: 189–194 [PubMed] [Google Scholar]
- 7. Payne WG, Murphy CG, Grossbard LJ. Combined laparoscopic and endoscopic approach to resection of gastric leiomyoma. J Laparoendosc Surg. 1995; 5: 119–122 [DOI] [PubMed] [Google Scholar]
- 8. Taniguchi E, Kamiike W, Yamanishi H, et al. Laparoscopic intragastric surgery for gastric leiomyoma. Surg Endosc. 1997; 11: 287–289 [DOI] [PubMed] [Google Scholar]
- 9. Tagaya N, Mikami H, Kogure H, Kubota K, Hosoya Y, Nagai H. Laparoscopic intragastric stapled resection of gastric submucosal tumors located near the esophagogastric junction. Surg Endosc. 2002; 16: 177–179 [DOI] [PubMed] [Google Scholar]
- 10. Seelig MH, Hinder RA, Floch NR, et al. Endo-organ and laparoscopic management of gastric leiomyomas. Surg Laparosc Endosc Percutan Tech. 1999; 9: 78–81 [PubMed] [Google Scholar]
- 11. Fujasaki J, Mine T, Akimoto K, Yoshidea S, Hasegawa Y, Ogata E. Enucleation of a gastric leiomyoma by combined laser and snare electrocuting technique. Gastrintest Endosc. 1988; 34: 128–130 [DOI] [PubMed] [Google Scholar]
- 12. Spinelli P, Cenai FG, Cambareri AR, Meroni E, Pizzatti P. Two step endoscopic resection of gastric leiomyomas. Surg Endosc. 1993; 7: 90–92 [DOI] [PubMed] [Google Scholar]
- 13. Gurbuz AT, Peetz ME. Resection of a gastric leiomyoma using combined laparoscopic and gastroscopic approach. Surg Endosc. 1997; 11: 285–286 [DOI] [PubMed] [Google Scholar]
- 14. Uchikoshi F, Ito T, Nishida T, Kitagawa T, Endo S, Matsuda H. Laparoscopic Intragastric Resection of gastric stromal tumor located at the esophago-cardiac junction. Surg Laparosc Endosc Percutan Tech. 2004; 14( 1): 1–4 [DOI] [PubMed] [Google Scholar]
- 15. Yamashita Y, Bekki F, Kakegawa T, Umetani H, Yatsuka K. Two Laparoscopic techniques for resection of leiomyomas in stomach. Surg Laparosc Endosc. 1995; 5: 38–42 [PubMed] [Google Scholar]
- 16. Tangoku A, Yamamoto K, Hirazawa K, et al. Laparoscopic resection of large leiomyomas of the gastric fundus. Surg Endosc. 1999; 13: 1050–1052 [DOI] [PubMed] [Google Scholar]