Abstract
Objective:
This study was designed to determine the relationship between interstitial cystitis (IC), endometriosis (endo), and chronic pelvic pain (CPP) in individuals in whom nongynecological and nonurological problems had been previously ruled out.
Methods:
A prospective study of 162 consecutive women with a complaint of chronic pelvic pain seen in the clinic was performed between August 2002 and December 2005. These patients underwent a workup to exclude other causes of pelvic pain, had PUF (Pain Urgency and Frequency) questionnaires filled out, and underwent a laparoscopy and a cystoscopy with hydrodistention. Pain levels were determined, and treatment was reviewed and enumerated. Results were obtained and quantified.
Results:
In this study, 123 (76%) patients were diagnosed with active endometriosis, 133 (82%) were diagnosed with interstitial cystitis, and 107 (66%) had both disease entities simultaneously. Thirteen (8%) patients were diagnosed with pathologies unrelated to endometriosis and interstitial cystitis. Pain levels were seen to decrease at 6 months in all groups of patients with the exception of those patients with endometriosis only.
Conclusions:
CPP is a difficult, taxing, and frustrating concern for many women in the United States. These individuals have traditionally been difficult to treat. A large number of women with CPP in our patient population have been shown to have endometriosis, interstitial cystitis, or both. Therefore, a workup for premenopausal individuals with CPP involves obtaining a history that keys into possible nongynecologic causes of pain, a complete accounting of urinary problems, and a thorough history of gynecological problems. A physical examination with a comprehensive history should be performed, and the investigation may include the possibility of a simultaneous laparoscopy and cystoscopy if warranted. These procedures can serve as both a means for diagnosis and treatment of these problems when encountered.
Keywords: Interstitial cystitis, Endometriosis, Chronic pelvic pain, Endoscopy
INTRODUCTION
Chronic pelvic pain (CPP) is a condition in which constant or intermittent pain of greater than 6-month duration is present in the pelvic area and lower abdomen. Its occur-rence affects >9 million women in the United States and is a major problem for individuals who have this problem.1 CPP is an illness that can debilitate many women and is linked with many additional associated problems. It is a difficult and complex diagnosis to make, and its detection is a frustrating concern for many women in the United States. It is thought that this disease state accounts for 10% to 15% of referrals to gynecologists2 and accounts for >40% of all diagnostic gynecological laparoscopies.3
It is believed that 25% to 40% or more of all patients who have had hysterectomies performed for CPP to end the discomfort continue to have pain after the procedure.4,5 These results suggest that in some individuals with CPP pain may originate from areas other than the uterus. A more defined study of patients who had previously had a hysterectomy for CPP without amelioration of the pain demonstrated that close to 80% had interstitial cystitis not previously detected.6 This suggests a need to investigate bladder dysfunction fully as the cause of CPP before suggesting to the patient that a hysterectomy will help their situation.
This was a prospective study including patients with CPP who had problems with other organ systems, such as gastrointestinal and musculoskeletal, ruled out. These patients were investigated for all gynecologic and urinary tract problems to determine the relationship between interstitial cystitis, endometriosis, and chronic pelvic pain in those patients without other organ system problems.
METHODS
From August 2002 to December 2005, 162 consecutive female, nonpostmenopausal patients with chronic pelvic pain were evaluated. Musculoskeletal, gastrointestinal, and other nongynecological and urological conditions were excluded with the patient being sent to appropriate specialists. A preliminary study has previously been reported.7 These patients were evaluated with detailed histories and physical examinations; the PUF questionnaire8; visual analog pain indices9–11; and investigation of any specific symptoms.
Physical examinations were used to correlate the location of the pain in comparison with other findings such as ovarian cysts or tender areas of the bladder. The examiners fingers were pressed on the anterior vaginal wall underlying the bladder in these individuals after they voided, to see whether they experienced a feeling of urgency or the need to urinate again. The rectal area was examined to determine whether pain or spasm occurred. The vaginal vault was digitally probed to feel for levator muscle pain or spasms, which often can be an important finding suggestive of an irritated bladder.12
All nongynecological and nonbladder sources of pain were ruled out. The number of patients who had been treated with NSAIDs (nonsteroidal anti-inflammatory medicines), narcotics, and other pain medications and the success with these medications was noted. In patients who were given ovulation suppression (76 patients) with oral contraceptive pills, the success and failure to reduce pain was noted. Ovulation suppression medications used before seeking help for pelvic pain were noted. These medicines included depot medroxyprogesterone (used in 7 patients for contraceptive purposes) and GnRH analogs (used on 14 patients to suppress ovarian function for treatment of their endometriosis and pain). Rates of success were documented. Urine cultures were performed to rule out bladder infections and where warranted, investigation of the upper urinary tract was done.
Laparoscopy was performed with a closed technique. The pelvis was evaluated and areas of pathology were recorded. Pathologic lesions were removed by available techniques including resection and fulguration of endometrial implants with the CO2 laser, electrosurgery, or Harmonic scalpel and cutting adhesions with the CO2 laser, Harmonic scalpel, and scissors. Diagnosis of endometriosis was made by pathological diagnosis obtained by biopsy or excision or by visual means of “typical” perito-neal lesions of a black charcoal appearance, as reports of pathological correlation in the diagnosis of endometriosis of up to 93% to 97% has been confirmed on peritoneal lesions with a typical black “powder burn” appearance.13,14
Cystoscopy was performed using sterile water as a dis-tending medium. Urinary structures including the urethra, urethral-vesicle junction, the trigone, dome, sidewalls of the bladder, and both ureteral orifices were evaluated upon first entry into the bladder. The bladder was allowed to equilibrate at a pressure of 80 cm of hydrostatic water pressure for 3 minutes to 4 minutes and then was allowed to slowly empty. Glomerulations were noted if visualized, and if >10 glomerulations per quadrant were present in at least 3 of the 4 quadrants in the bladder, a diagnosis of IC was made (for the purposes of this study). The bladder was allowed to drain at the conclusion of the procedure.
Within this group of 162 patients, 8 were less than 18 years old. They had investigations similar to all the patients including laparoscopy and cystoscopy. Two of the patients began having pain at age 12, approximately 3 months to 4 months after beginning menses; in 3 others their discomfort began at age 15 to 16 shortly after beginning menses. One patient had had 5 laparoscopies with a postoperative diagnosis of endometriosis each time and had been referred to a major medical pediatric pain clinic without a clear diagnosis for her discomfort being established. All these adolescent patients had IC established as part of their pain complex with the appropriate questions, examinations, and procedures.
Pain levels were assessed by use of a visual analog scale before initial treatment, immediately after treatment, after 3 months, after 6 months, and after 1 year. In patients who had a diagnosis of endometriosis and had fulguration of endometriosis or excision of the endometriotic lesions at the time of laparoscopy, 34% had treatment with GnRH agonists or medroxyprogesterone after the laparoscopic procedure. Of the 16 patients who had a diagnosis of endometriosis for their CPP without other evident causes, 7 had further treatment of their mild to moderate disease. Patients with interstitial cystitis133 had their IC treated after cystoscopy with PPS (pentosan polysulfate sodium).
All statistical analyses were performed with SAS 9.1 software. Pair-wise comparisons were performed with the Student t, Bonferroni, Scheffé, and Tukey-Kramer multicomparison methods. For testing the effect of pain treatment, we fitted proportional odds modeling to patient data.
RESULTS
Of the 162 women with a complaint of chronic pelvic pain, 123 (76%) were diagnosed with endometriosis and treated with fulguration of the endometriosis, medical treatment after the surgical procedure, or both (Table 1). Thirty-three of these patients (26%) had a previous history of endometriosis. Sixteen patients (10%) were diagnosed only with endometriosis and no other pathology (Table 1).
Table 1.
Pain Urgency and Frequency (PUF) Scores and Pain Levels (Visual Analog Scale) for Patients With Chronic Pelvic Pain
| Patients* | N | % | Mean PUF Score | Initial Pain (010) | Dysmenorrhea (0-10) | Dyspareunia (0-10) | Pain After Surgery (0-10) | Pain 6 Months After Surgery (0-10) |
|---|---|---|---|---|---|---|---|---|
| All IC patients | 133 | 82 | 21.2 | 5.4 | 8.1 | 7.3 | 1.2 | 3.2 |
| IC and endo | 107 | 66 | 20.8 | 5.3 | 8.0 | 7.0 | 1.5 | 2.7 |
| All endo patients | 123 | 76 | 19.6 | 5.2 | 7.8 | 6.5 | 1.3 | 2.7 |
| IC only | 26 | 16 | 22.6 | 5.5 | 8.0 | 7.5 | 1.5 | 3.2 |
| Endo only | 16 | 10 | 12.0 | 5.1 | 7.6 | 6.6 | 1.1 | 1.9 |
| Other pathology | 13 | 8 | 7.5 | 3.2 | 5.6 | 2.5 | 0.5 | 1.1 |
IC = interstitial cystitis patients; endo = endometriosis patients.
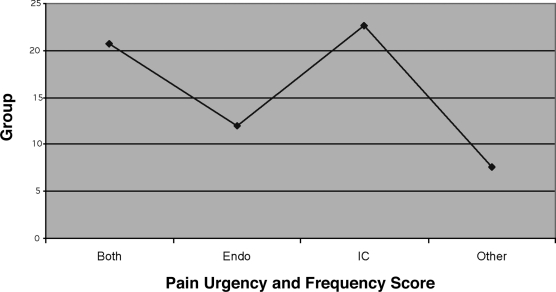
Interstitial cystitis was present in 133 (82%) patients. Of these, 96 (72%) had complained of lower urinary tract symptoms. Twenty-six (16%) patients were diagnosed with IC and no endometriosis was seen. Correlation with an elevated PUF score was also made (Figure 1).
Figure 1.
Mean PUF scores.
In 107 (66%) patients, both endometriosis and interstitial cystitis were found at the same time. These patients were treated for their endometriosis with excision or fulguration of the endometriosis at laparoscopy, medication after the surgery, or both modalities. Cystoscopy and hydro-distention at the time of cystoscopy was the initial treatment of IC for symptoms, and after surgery these individuals were treated with PPS and symptomatically if necessary with anticholinergic medications, antihistamines, or other appropriate medication. Other treatment modalities, such as intravesicle instillations, were performed as needed for painful bladder type syndrome.
Thirteen patients (8%) had no pathology or pathology different than endometriosis and interstitial cystitis. This included 3 patients with an ovarian cyst, 2 with ovarian remnant syndrome; 2 with only adhesions, 2 with a combination of adhesions and ovarian cysts, and 4 with no pathology seen (all had PUF scores <20).
Of those patients who had not had a hysterectomy, 83% (97 patients) had a worsening of symptoms around their period. Of these, 12 had endometriosis as the only definable pathology, 15 had interstitial cystitis alone, and 70 had both endometriosis and interstitial cystitis.
Before the workup, 76 patients had been on oral contraceptives, and of these 52 (68%) had had no amelioration of their pain. Of these, 63 (83%) had endometriosis, 70 (92%) had interstitial cystitis, and 53 (71%) had both. Fourteen patients had been on GnRh analogs to treat endometriosis pain, and 12 of these individuals either had no pain relief, or after a short course of treatment, the pain resumed while the patients were on the medicine or had recently finished it.
PUF scores (Figure 2) were determined for groups consisting of 1) both IC and endometriosis (BOTH), 2) IC alone (IC), 3) endometriosis alone (Endo) and 4) other conditions found with the patients with CPP (Other). All groups had statistically different PUF scores.
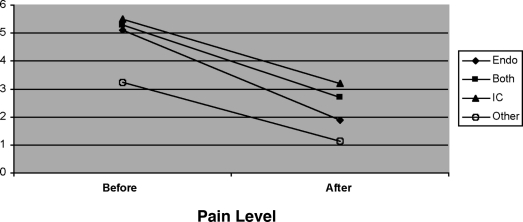
Figure 2.
Average changes in pain before and six months after surgery (visual analog scale).
Statistical analysis utilizing pair-wise comparisons demonstrated that the PUF scores were similar in certain groups, and a trend was seen in other groups. The Student t test demonstrated that mean PUF scores of the group consisting of both IC and endometriosis (BOTH) and the group consisting of IC patients only are statistically the same. The mean PUF score of the BOTH group is greater than that of the group consisting of patients with endometriosis only and that consisting of other factors such as ovarian cysts (OTHER group). Similarly, the mean PUF score of the ENDO group is greater than that of the OTHER group but less than the PUF scores of the IC only group. The IC group PUF score is greater than that of the PUF score of the OTHER group (ie, PUF scores of the BOTH group = the PUF scores of the IC group and is greater than that of the endometriosis only group (ENDO), which is greater than the PUF scores of the OTHER group).
The Bonferroni, Scheffé, and Tukey-Kramer multicomparison tests demonstrate that the mean PUF scores of the BOTH group and the IC group are the same, and the mean PUF scores of the ENDO & OTHER group are the same. The mean PUF score of the BOTH group is greater than that of the ENDO group. Similarly, the mean PUF score of the ENDO group is less than the IC group PUF score. The IC group PUF score is greater than that of the OTHER group. This means that the mean PUF scores of the group with both IC and endometriosis (BOTH) are statistically equivalent to those of the group with interstitial cystitis alone (IC) and both are greater than that of the groups with endometriosis alone (ENDO) and the group having other pathology (OTHER).
On physical examination, palpation of the bladder through the vaginal wall was performed on all patients. The great majority of those with IC had sensitive or painful experiences with this procedure. Often during treatment, as the generalized pain and discomfort of the patient improved, the tenderness and feeling of having to void would disappear.
Of the patients less than 18 years old, 7 of the 8 had endometriosis and 6 had interstitial cystitis. Pain relief was dramatic in all initially, and treatment was performed for the entire course of treatment, even when the pain abated; but after a year pain recurred in 3 of the patients.
Pain scores using the visual analog scale were initially determined, and then recalculated after surgery at 3 months after surgery and 6 months after surgery (Table 1). The PUF scores were compared with the pain scores. At the postoperative visit, 150/162 reported that pain was either gone or had been significantly improved. After 3 months, 120/162 had tremendous amelioration of the pain that was present before laparoscopy, after removal of pathology, and cystoscopy with hydro-distension. At 6 months, a decrease in pain was noted compared with the level of pain before surgery (Figure 1). The proportional odds ratio modeling estimate statistics were performed at a level where the odds of ranking pain at each level after surgery demonstrate a 93.4% probability. This means that the chance of having a pain level less than that before surgery was present for the groups consisting of BOTH (a group consisting of patients having both IC and endometriosis), IC (the group consisting of IC patients only), and the group consisting of patients with other problems (the OTHER group). Statistical analysis of the group with only endometriosis found as the pathology demonstrated that treatment was not effective in reducing the pain for a long duration.
At 1 year, only 71%115 had pain levels less than those before surgery. During this time, treatment was initiated for endometriosis if agreed upon by the patient, and vigorous treatment of the IC was performed by the patient taking pentosan polysulfate sodium (Elmiron) and having adjuvant treatments.
DISCUSSION
A major problem for many woman in the United States is chronic pelvic pain. It causes not only quality of life issues but is also responsible for excessive costs to our society. Patients with chronic pelvic pain have traditionally been difficult to manage. This is due in part to the difficulty in making a correct diagnosis and is further exacerbated by the long list of possible differential diagnoses that exist for this disease. It has been seen that a large number of woman complaining of CPP have endometriosis, interstitial cystitis, and more recently, we have seen both together. Is there a strong causal relationship between interstitial cystitis and endometriosis? The cause of endometriosis is unknown, as is the cause of IC. Therefore, more research is necessary to delineate the causes and the relationship between the 2.
The literature suggests that the major causes of pelvic pain of a chronic nature have been due to disease states including1 gynecological problems like endometriosis, adhesions, pelvic inflammatory disease (PID), leiomyomata, and adenomyosis2; gastrointestinal disease3; skeletal disorders; and4 genitourinary problems. After ruling out nongynecological and nongenitourinary disorders, laparoscopies in those patients with chronic pelvic pain have demonstrated endometriosis in 30% to 90% of these individuals, adhesions in up to 50%, chronic pelvic inflammatory disease was seen in a modest number of the patients, and some patients had no pathology.15–20 Patients in whom no pathological cause was noted have frequently been told that the source of their pain was psychological or that removing the uterus would provide a definitive cure of the problem. This has obviously not been the truth. Some reports21 have indicated that up to 38% of individuals with CPP had interstitial cystitis as a component of their disease. Recent studies in patients with nonurogynecological causes of their CPP ruled out have seen a high correlation between patients with CPP and endometriosis and interstitial cystitis.7,22,23 In these peer-reviewed papers, the percentage of patients having both interstitial cystitis and endometriosis was 60% to 66%, and the number of women having both interstitial cystitis and endometriosis or one or the other pathology was over 90%.
Lentz et al24 have described patients with known interstitial cystitis who experienced menstrual cycle-related changes of their interstitial cystitis symptoms and subsequently had a laparoscopy and cystoscopy with hydro-distension performed. Endometriosis was found in the majority of these patients. The authors suggest that, “diagnostic laparoscopy should be considered together with hydro-distension of the bladder” in those patients in whom pelvic pain and lower urinary symptoms are “exacerbated premenstrually.” They demonstrated a relationship between known interstitial cystitis and endometriosis in this subgroup of patients with chronic pelvic pain.
Several authors7,22,23 have described individuals with CPP who underwent laparoscopy and cystoscopy, and they demonstrate a relationship between endometriosis and interstitial cystitis. Chung22 described a subset of patients who had undergone hysterectomy for pelvic pain without amelioration of the pain, and a large number had interstitial cystitis in addition to their previously diagnosed endometriosis. Parsons et al25 showed that his potassium sensitivity test (PST) was positive in 81% of women with chronic pelvic pain and appeared to correlate to clinically diagnosed gynecologic disease states, such as endometriosis (86%), vulvodynia (79%), and interstitial cystitis (100%).26
It is known that a genetic tendency for endometriosis exists within families, but the possibility of a genetic predisposition within families to interstitial cystitis has not yet been demonstrated. In our study, 35 women stated that they did not think that the increased lower urinary tract symptoms were anything but normal. “My mother had a weak bladder and so do I” was not an uncommon comment. We were able to find 9 sets of mothers and daughters who had both IC and endometriosis, although the symptoms and amount of pain was different in each. Two sets of mothers, daughters, and grandmothers were examined. The grandmothers were postmenopausal and, therefore, had only IC diagnosed; but the mothers and daughters both had IC and endometriosis.
Chronic pelvic pain is a difficult, taxing, and frustrating concern for many women in the United States. These individuals have traditionally been difficult to treat effectively. A large number of women with CPP in our patient population have been shown to have endometriosis, interstitial cystitis, or both. Therefore, a workup for premenopausal individuals with CPP involves obtaining a good history and physical. This allows the clinician to develop a good differential diagnosis of nongynecologic causes of pain, urinary problems, and gynecological problems. PUF questionnaires correlate well to those patients with interstitial cystitis, but the results do not show a perfect correlation. Some patients who had high levels on their PUF questionnaire did not have a cystoscopic correlation. These individuals did respond well to treatment of their bladder dysfunction treated as if it were IC. A good physical examination should rule out vaginal vault discomfort and a tender bladder on vaginal examination; these particular points are important features of painful bladder syndrome. In the investigation, a simultaneous laparoscopy and cystoscopy can be performed if warranted. These procedures can serve as both a means for diagnosis and partial treatment regimen when endometriosis, interstitial cystitis, or both, are encountered.
Just because someone has a disease state found in the workup does not mean that that entity is the cause of the pain. Adhesions in patients with CPP may not be the causative agent for their pain.27 Endometriosis can be found in patients with problems other than pain,28 and because the symptoms of pain may exist in those individuals without endometriosis, the relationship between endometriosis and pain is uncertain. It is accepted that patients with endometriosis can have discomfort or may be free of pain. How do we know if the pain the individual is experiencing is due to the endometriosis? Hurd29 suggests that 3 criteria be met before attributing the chronic pain to endometriosis: the pelvic pain should be cyclical, treatment should be accompanied by long-term relief, and the endometriosis should be diagnosed surgically, not by symptoms. Almost all of our patients with endometriosis had cyclical pain and were diagnosed by laparoscopy; however, the majority of those with endometriosis as the only known component to their CPP did not statistically have long-term relief from their pain.
Although endometriosis most commonly presents as dysmenorrhea, endometriosis-associated discomfort can occur with problems like ovulation, pain associated with adhesions, painful bowel movements, and secondary to inflammatory reactions in the pelvis. The most complex question is when does endometriosis cause pelvic pain and is the problem in these patients with endometriosis connected to CPP? Although the numbers of patients having pain associated with endometriosis only are small, this study demonstrates that the long-term effectiveness of treatment for pain secondary to endometriosis is not good. Obviously, some patients benefit from treatment of the disease, but many of these patients do not seem to have long-term amelioration of their symptoms, making it difficult to know whether the endometriosis is a contributing factor in the pain in all patients with endometriosis. One has to focus on the need to diagnose and treat as completely as possible interstitial cystitis as an entity of chronic pelvic pain. If a patient in her reproductive years has interstitial cystitis, then it may be necessary to look for endometriosis and vice-versa if the conditions warrant.
Chronic pelvic pain can be a difficult, frustrating process to diagnose accurately. To pursue a course of action for the patient, a physician must rule out the many potential causes of the pain. If one is able to rule out nongyneco-logical and nonurological problems, then keying in on situations where the symptoms will lead the practitioner to the source will allow for treatment.30
Contributor Information
John D. Paulson, Washington Area Reproductive and Urogynecology Services, Reston, Virginia, USA..
Melissa Delgado, Blue Ridge Ob/Gyn, Culpepper, Virginia, USA..
References:
- 1. Mathias SD, Kupperman M, Lieberman RF, Lipschutz RC, Steege JF. Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates. Obstet Gynecol. 1996;87(3):321–327 [DOI] [PubMed] [Google Scholar]
- 2. Reiter RC. A profile of women with chronic pelvic pain. Clin Obstet Gynecol. 1990;33(1):130–136 [PubMed] [Google Scholar]
- 3. Howard FM. The role of laparoscopy in chronic pelvic pain: promise and pitfalls. Obstet Gynecol Surv. 1993;48:357–387 [DOI] [PubMed] [Google Scholar]
- 4. Stovall TG, Ling FW, Crawford DA. Hysterectomy for chronic pelvic pain of presumed uterine etiology. Obstet Gynecol. 1990;75(4):676–679 [PubMed] [Google Scholar]
- 5. Hillis SD, Marchbanks PA, Peterson HB. The effectiveness of hysterectomy for chronic pelvic pain. Obstet Gynecol. 1995;86(6):941–945 [DOI] [PubMed] [Google Scholar]
- 6. Chung MK. Interstitial cystitis in persistent posthysterectomy chronic pelvic pain. JSLS. 2004;8:329–333 [PMC free article] [PubMed] [Google Scholar]
- 7. Paulson JD, Delgado M. Chronic pelvic pain: the occurrence of interstitial cystitis in a gynecological population. JSLS. 2005;9:426–430 [PMC free article] [PubMed] [Google Scholar]
- 8. Parsons CL, Dell J, Stanford EJ, et al. Increased prevalence of interstitial cystitis: previously unrecognized urologic and gynecologic cases identified using a new symptom questionnaire and intravesical potassium sensitivity. Urology. 2002;60(4):573–578 [DOI] [PubMed] [Google Scholar]
- 9. Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485–489 [DOI] [PubMed] [Google Scholar]
- 10. Averbach M, Katzper M. Assessment of visual analog versus categorical scale for measurement of osteoarthritis pain. J Clin Pharm. 2004;44:368–372 [DOI] [PubMed] [Google Scholar]
- 11. Acute pain management: operative or medical procedures and trauma, clinical practice guideline No. 1. AHCPR Publication No. 92-0032; February 1992. Agency for Healthcare Research & Quality, Rockville, MD; 116–117 [Google Scholar]
- 12. Howard FM, Perry CP, Carter JE, El-Minawi AM. Pelvic pain: diagnosis and management. Philadelphia, PA: Lippincott; 2000; 35–39 [Google Scholar]
- 13. Stripling MC, Martin DC, Chatman DL, Zwaag RV, Poston WM. Subtle appearance of pelvic endometriosis. Fertil Steril. 1988;49(3):427–431 [DOI] [PubMed] [Google Scholar]
- 14. Nisolle M, Paindaveine B, Bourdon A, Berliere M, CasanasRoux F, Donnez J. Histologic study of peritoneal endometriosis in infertile women. Fertil Steril. 1990;53:984–988 [DOI] [PubMed] [Google Scholar]
- 15. Howard FM. The role of laparoscopy as a diagnostic tool in chronic pelvic pain. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(3):467–494 [DOI] [PubMed] [Google Scholar]
- 16. Kontoravdis A, Hassan E, Hassiakos D, Kontoravdis N, Creatsas G. Laparoscopic evaluation and management of chronic pelvic pain during adolescence. Clin Exp Obstet Gynecol. 1999;26:76–77 [PubMed] [Google Scholar]
- 17. Carter JE. Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J Am Assoc Gyn Laparosc. 1994;2:43–47 [DOI] [PubMed] [Google Scholar]
- 18. Bynyavejchevin S, Rungruxsirivorn T, Pinchantra P, Wisawasukmongchol W, Suivajanakorn S, Limpaphayom K. Laparoscopic findings in Thai women with chronic pelvic pain. J Med Assoc Thai. 86(supp l2):S404–S408, 2003 [PubMed] [Google Scholar]
- 19. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol. 1998;92:1029–1032 [DOI] [PubMed] [Google Scholar]
- 20. Cunanan RG, Courey NG, Lippes J. Laparoscopic findings in patients with pelvic pain. Am J Obstet Gynecol. 1983;146:589–591 [DOI] [PubMed] [Google Scholar]
- 21. Myers DL, Aguilar VC. Gynecologic manifestations of inter-stitial cystitis. Clin Obstet Gynecol. 2002;45(1):233–241 [DOI] [PubMed] [Google Scholar]
- 22. Chung MK, Chung RP, Gordon D, Jennings C. The evil twins of chronic pelvic pain syndrome: endometriosis and interstitial cystitis. JSLS. 2002;6:311–314 [PMC free article] [PubMed] [Google Scholar]
- 23. Chung MK, Chung RP, Gordon D. Interstitial cystitis and endometriosis in patients with chronic pelvic pain: the “evil twins” syndrome. JSLS. 2005;9:25–29 [PMC free article] [PubMed] [Google Scholar]
- 24. Lentz GM, Bavendam T, Stenchever MA, Miller JL, Small-dridge J. Hormonal manipulation in women with chronic, cyclic irritable bladder symptoms and pelvic pain. Am J Obstet Gynecol. 2002;186(6):1268–1273 [DOI] [PubMed] [Google Scholar]
- 25. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395–1400 [DOI] [PubMed] [Google Scholar]
- 26. Parsons CL, Bullen M, Kahn BS, Stanford DJ, Willems JJ. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98(1): 127–132 [DOI] [PubMed] [Google Scholar]
- 27. Hammoud A, Gago LA, Diamond MP. Adhesions in patients with chronic pelvic pain: a role for adhesiolysis? Fertil Steril. 2004;82(6):1483–1491 [DOI] [PubMed] [Google Scholar]
- 28. Fauconniier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595–606 [DOI] [PubMed] [Google Scholar]
- 29. Hurd WW. Criteria that indicate endometriosis is the cause of chronic pelvic pain. Obstet Gynecol. 1998;92:1029–1032 [DOI] [PubMed] [Google Scholar]
- 30. Paulson JD, Gor HB. Management of chronic pelvic pain. Expert Rev Obstet Gynecol. 2007;2(1):37–50 [Google Scholar]