Abstract
Objective:
We assessed a unique technique of laparoscopic peritoneal dialysis (PD) catheter insertion which can minimize catheter dysfunction.
Methods:
We performed a retrospective review of patients undergoing laparoscopic PD catheter placement with a Quinton percutaneous insertion kit between July 2000 and December 2004.
Results:
Thirty-one catheters were placed laparoscopically. The mean operating time was 52 minutes. Adhesiolysis was required in 9 (29%) and omentectomy or omen-topexy in 3 (10%) cases. Late complications included catheter dysfunction in 2 patients (6.5%), debilitating abdominal pain requiring catheter removal in 1 patient, and 1 trocar-site hernia. The mean follow-up was 17 months.
Conclusions:
Laparoscopic PD catheter insertion using a Quinton percutaneous insertion kit is safe, reproducible, and effective. It facilitates placement of the catheter tip into the pelvis and allows adhesiolysis, omentectomy, or omentopexy when necessary. Utilization of this technique results in a low rate of PD catheter dysfunction.
Keywords: Minimally invasive surgery, Laparoscopy, Continuous ambulatory peritoneal dialysis, Catheter, In-dwelling, Catheter dysfunction
INTRODUCTION
Continuous ambulatory peritoneal dialysis (CAPD) has become a widespread mode of dialysis for patients with chronic renal failure. The surgeon's role in caring for these patients is to provide access to the peritoneal cavity via a peritoneal dialysis (PD) catheter and to diagnose and treat catheter complications.
In 1968, Tenckhoff and Schechter described a percutaneous nonvisualized method of catheter placement. However, this was associated with a risk of bowel or vessel injury, as well as a high incidence of malpositioned catheters resulting in failure rates of up to 65% at 2 years.1 Subsequently, the gold standard became open placement under direct surgical vision via minilaparotomy.2 However, placement of the catheter tip into the pelvis is essentially a blind technique. This technique has resulted in up to a 22% incidence of drainage dysfunction.3 Two major factors that may be involved in catheter dysfunction are inadequate placement of the catheter tip into the pelvis, which allows the catheter to migrate and become entrapped within the omentum, and the presence of intraabdominal adhesions, which interfere with correct catheter placement and may cause the PD fluid to locu-late.3–6
In an attempt to improve catheter function and decrease complications, in 1981 Ash et al7 reported on a peritoneoscopic technique. He used a special needlescope (YTEC, Medigroup, Inc., North Aurora, IL) with surrounding cannula and catheter guide. This method reduced the early failure rate to 3% in his hands. However, it does not allow for adhesiolysis, and furthermore, it requires specialized equipment.
Over the last decade, several reports have described laparoscopic8–17 or minilaparoscopic18–20 placement of PD catheters. This approach addresses many concerns by allowing direct visualization of the peritoneal cavity and exact placement of the catheter tip deep into the pouch of Douglas. It also allows laparoscopic adhesiolysis and omentopexy or omentectomy. We developed a 2-port minilaparoscopic technique of PD catheter insertion, using a Quinton percutaneous insertion kit (Tyco Healthcare Group LP, Mansfield, MA). We hypothesize that this technique has a low complication rate, results in a low rate of catheter dysfunction, and should be easily adopted into general and laparoscopic surgery practice. We report our first 31 laparoscopic catheter placements.
METHODS
A retrospective review was performed of patients who underwent laparoscopic PD catheter insertion by the primary author (SPH) or one of his 2 partners over a 4-year period. All patients were followed regularly by one clinical nurse specialist (MC).
Surgical Technique
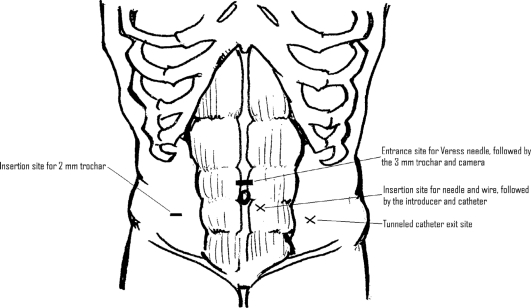
The exit site in the left lower abdomen is marked taking into consideration the belt line in the preoperative holding area with the patient standing (Figure 1). The patient is subsequently placed on the operating room table in a supine position with both arms tucked, and general anesthesia is administered. Perioperative prophylactic intravenous antibiotics are administered, and an Ioban is placed on the abdomen after it is prepped in a sterile fashion. The optimal trocar placement is shown in Figure 1. A Veress needle is used to establish pneumoperitoneum. In the presence of a prior incision, an open insertion technique is utilized. Some have argued that a midline port for the scope is too close to the insertion point, but we have had no trouble with visualization if the sheath is pulled back near the fascia.
Figure 1.
Port placements and catheter insertion and exit sites.
We initially used a 5-mm trocar and zero-degree laparoscope for the first 5 cases. We then switched to a 3-mm metal port and a 2.7-mm laparoscope (Stryker Endoscopy, San Jose, CA) for the remaining cases to reduce the risk of development of an incisional hernia postoperatively. A 2-mm Miniport (Autosuture, US Surgical Corp., Norwalk, CT) is then placed in the right lower abdomen lateral to the rectus sheath (Figure 1) under direct visualization if no adhesions are present. If adhesions are present, adhesiolysis is accomplished by using either Endoshears or the Harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH) through 5-mm trocars.
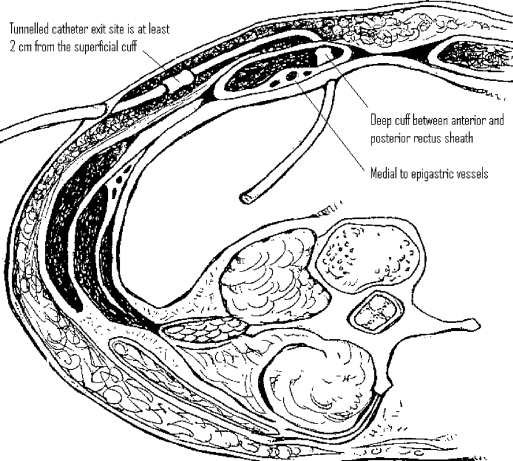
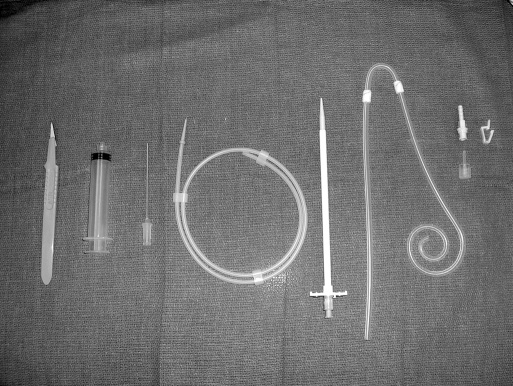
Once the pelvis is free, attention is turned to the anterior abdominal wall. The entrance and exit sites are marked (Figure 1). It is helpful to lay the catheter on the abdomen to estimate the entrance site based on the length of the patient's torso. The tip should easily reach the cul-desac, thus the top of the curl should be at the pubic symphysis. The author prefers an entrance site within the rectus sheath medial to the epigastric vessels (Figure 2). The exit site is identified once again. In patients who are kidney transplant candidates, it is preferable to use the left side of the abdomen. We use a Quinton insertion kit, which contains a swan neck Curl Cath double-cuffed PD catheter, a 16-French Pull-Apart introducer, an 18-gauge introducer needle, a 10-cc syringe, a J/straight guidewire, tunneling stylet, #11 scalpel, gauze sponges, Beta-Cap adapter, cap, clamp, and instructions (Figure 3). A 1-cm incision is made at the insertion site, and the needle is inserted through the abdominal wall under direct laparoscopic vision. The needle is oriented obliquely to position the catheter in a caudad direction, making it less likely to migrate into the upper abdomen in the future. The wire is advanced through the needle into the pelvis. The needle is removed, and the sheath and dilator are inserted over the wire by using the Seldinger technique. The dilator is removed, and the peritoneal dialysis catheter is fed through the sheath toward the pelvis. Once the catheter is inside the abdomen, the sheath is pulled apart leaving the catheter in place. The external end is tunneled and pulled through an exit site lateral to the insertion site. It is important that the distal cuff is greater than 2cm from the skin incision. The proximal or internal cuff is then buried under the anterior rectus sheath by using a hemostat (Figure 2). The entrance site is inspected internally using the laparoscope to verify that the cuff is not advanced through the posterior sheath into the abdomen.
Figure 2.
Cross section showing optimal catheter placement through abdominal wall.
Figure 3.
Contents of the Quinton insertion kit: #11 scalpel, 10-cc syringe, 18-gauge introducer needle and 10-cc syringe, J/straight guidewire, 16-French Pull-Apart introducer, Swan neck curl cath, double-cuffed PD catheter, beta-cap adapter, cap, clamp, and instructions
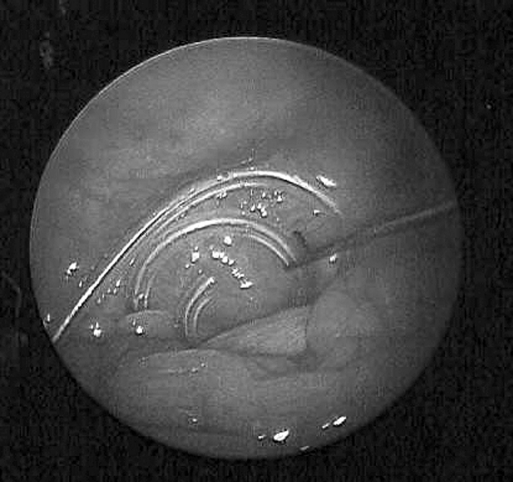
The 2-mm grasper is inserted and used to position the curled portion of the catheter deep into the pelvic cul-desac. When positioned correctly, the coiled end is barely visible above the small bowel (Figure 4). If omentopexy is necessary, it is done using the Endo Close (US Surgical Corp, Norwalk, CT) as described by Crabtree et al.16 Omentectomy is performed using the Harmonic scalpel.
Figure 4.
Two-mm grasper advancing the curl catheter into the pelvic cul-de-sac.
Once the procedure is completed, the trocars are removed and the pneumoperitoneum is evacuated. If a 5-mm or greater trocar is used in the midline, the fascia is closed with a 2.0 Vicryl suture. The PD catheter is then tested by infusing 250cc of saline into the abdomen and then draining it. If the catheter is functioning properly, it is locked with heparin 100μ/cc. The catheter is used no sooner than 2 weeks later.
RESULTS
Thirty-three attempts were made at laparoscopic PD catheter insertion between July 2000 and December 2004 at Highland Park Hospital. Two patients (6%) required conversion to a laparotomy. The first patient had dense adhesions that did not allow access to the peritoneal cavity, and the other developed severe omental bleeding after adhesiolysis. Neither of these is included in our outcome data. Thirty-one PD catheters were successfully placed using a Quinton percutaneous insertion kit in 30 patients (One patient had her catheter removed for peritonitis. A second was placed laparoscopically 2 months later.). The mean age was 57 years (range, 36 to 74), and they were evenly distributed between male and female. The causes of renal failure in our population are shown in Table 1. Eighteen patients (60%) had prior abdominal operations.
Table 1.
Causes of renal failure
| Number | Percent | Disease |
|---|---|---|
| 10 | 30% | Diabetes and hypertension |
| 5 | 16.7% | Hypertension |
| 3 | 10% | Diabetes |
| 3 | 10% | Glomerulonephritis |
| 2 | 6.7% | Polycystic kidney disease |
| 1 | 3.3% | Acute tubular necrosis |
| 1 | 3.3% | Systemic lupus erythematosus |
| 1 | 3.3% | Medication induced nephropathy |
| 1 | 3.3% | Renal artery stenosis |
| 1 | 3.3% | Renal cell carcinoma |
| 1 | 3.3% | Wegener's granulomatosis |
| 1 | 3.3% | Hemochromatosis |
The average operative time was 52 minutes for all patients (range, 18 to 161) but was 36 minutes for patients who only had insertion of a PD catheter. Fifteen patients had concomitant procedures, including lysis of adhesions in 9 patients (29%), omentopexy in 2 patients (6.5%), and omentectomy in 1 (3%) (Table 2). No visceral or vascular injuries occurred. Patients were discharged on the same day in 77% or the next day in 19% of cases. The average time between catheter placement and commencement of peritoneal dialysis was 51 days (range, 23 to 143).
Table 2.
Procedures performed in combination.
| Number | Percent | Procedure |
|---|---|---|
| 9 | 29% | Lysis of adhesions |
| 5 | 16% | Umbilical hernia repair |
| 2 | 6.5% | Inguinal hernia repair with mesh |
| 2 | 6.5% | Omentopexy |
| 1 | 3.2% | Omentectomy |
| 1 | 3.2% | Ventral hernia repair |
Perioperative cellulitis developed at the exit site in one patient but resolved with oral antibiotic therapy. Complications are listed in Table 3. Two patients (6.5%) had catheter malfunction at 1 month and 4 months after initiating CAPD. They both underwent laparoscopic revision of the catheter. In the first of these patients, severe intra-abdominal adhesions from a prior kidney pancreas transplant prevented effective dialysis even after revision. The latter catheter functioned well after revision that included omentectomy but was later removed due to refractory peritonitis. Peritonitis occurred in 2 patients (6.5%), and both patients required removal of the catheter; one patient had a second catheter placed at a later date.
Table 3.
Late complications.
| Number | Percent | Complication |
|---|---|---|
| 2 | 6.5% | Catheter dysfunction |
| 2 | 6.5% | Peritonitis |
| 1 | 3.2% | Umbilical hernia |
| 1 | 3.2% | Recurrent left inguinal hernia |
| 1 | 3.2% | Debilitating abdominal pain |
| 1 | 3.2% | Intractable hypoglycemia |
One patient in our series developed debilitating abdominal pain while another requested a switch to hemodialysis and both underwent catheter removal. One patient developed a recurrent inguinal hernia and one developed an umbilical trocar site hernia. We have not had any incisional hernias develop since we began using 3-mm trocars and closing midline trocar defects 5mm or larger.
The catheters were functional for an average of 14 months (range, 1 to 41). Six patients (19%) have died secondary to causes unrelated to the PD catheter. One patient was lost to follow-up. Table 4 summarizes the outcomes in patients who remain alive. Sixty-three percent of the catheters are still functioning. Of the patients whose catheters were removed, 3 (10%) have undergone successful renal transplantation, 2 developed peritonitis, 1 had intractable pain, and 1 had a return of his native kidney function. Mean follow-up in our series was 17 months (range, 2 to 41).
Table 4.
Catheter disposition in alive patients.
| Number | Percent | Procedure |
|---|---|---|
| 15 | 63% | Remain on PD |
| 3 | 13% | Underwent renal transplantation |
| 2 | 8% | Removed for peritonitis |
| 1 | 4% | Removed for intractable pain |
| 1 | 4% | Removed for catheter dysfunction |
| 1 | 4% | Removed because renal failure resolved |
| 1 | 4% | Lost to follow-up |
DISCUSSION
Laparoscopic insertion of PD catheters is an innovative use of minimally invasive surgery. During the last 12 years, several reports have been published regarding laparoscopic techniques to facilitate the placement of PD catheters.8–20 Our operation is unique in that it is a 3-puncture technique using minilaparoscopic instruments and the Quinton sheath and dilator to place the catheter through the abdominal wall. We believe the second port, which in our case is a 2-mm miniport, is important to allow manipulation and exact placement of the coiled end of the catheter deep into the pelvis. This location seems to allow better drainage and fewer problems with migration or omental wrapping. Those who use a port for the scope and one for the catheter are not routinely able to get the catheter in this deep location. We needed to convert to open in 6% of patients, which compares favorably with a 5.2% conversion rate for laparoscopic cholecystectomy and 21% conversion rate for laparoscopic colectomy.21,22 Because this is a study following our patients who underwent laparoscopic PD catheter insertion, we did not include these 2 in the data analysis.
In prior reports, various insertion techniques and port placements were used, and the dysfunction rates ranged from 0% to 14%. One recent report utilized the Quinton percutaneous insertion kit using a 1-port technique.17 However, they use a 10-mm camera port, which is prone to herniation, and they fix the catheter to the abdominal wall, which we feel is unnecessary. They report 13 patients with a 7.6% incidence of catheter migration and a 15% leak rate. In addition, Watson et al10,14 adopted a sheath and dilator technique for insertion. However, they have a drastically different technique using 5-mm and 11-mm trocars to allow suture fixation of the catheter into the pelvis. In addition, they report that 14% of the catheters malfunctioned, and 7% of patients developed port-site hernias. We did not fix the catheter to the pelvis with sutures and report a lower incidence of drainage dysfunction; therefore, we do not advocate fixing the catheter in the pelvis. Suturing the catheter to the bladder can add the risk of internal herniation of the small bowel.
In most prior reports,8–10,12–15 a combination of 5-mm and 10-mm ports were used, and the catheter was fed through one port, while one author used an 8-mm port to insert the catheter.11,16 We believe that insertion through the sheath is easier and more effective because the fascial defect is only as large as the catheter. This, along with using 2-mm and 3-mm ports, helps avoid herniation and fluid leak. We allow our patients to start PD 2 weeks after insertion, but due to referral patterns by the nephrologists, our patients began PD 51 days after insertion. The average time to initiation of PD is now shorter because of their understanding of our technique.
Varela et al19 described a minilaparoscopy technique using a modified Seldinger technique with sheath and dilator. They used 2-mm ports and a 1.9-mm laparoscope. However, they only reported a pilot study of 7 patients and did not present follow-up data.19 We have shown that our described technique using conventional laparoscopic equipment, miniports, and the Quinton percutaneous insertion kit is simple, reproducible, and safe, with no major perioperative complications.
This study reveals a low incidence of catheter dysfunction. Twenty-nine patients (93%) had a functional PD catheter for an average of 14 months. In one kidney-pancreas transplant patient with multiple dense adhesions, the catheter never worked normally and revision was unsuccessful. One other patient, who did not have an omentopexy, required revision secondary to omental trapping one month after the initial surgery. After laparoscopic revision and omentectomy, the patient's catheter worked well for 15 months.
To avoid omental trapping, it has been proposed that omentectomy be performed at the time of catheter insertion.23 However, this does add the potential morbidity of bleeding and prolongs operative time. Given the results of our experience, we prefer to avoid routine omentectomy. We would, however, perform a laparoscopic omentectomy if a patient underwent re-exploration for catheter dysfunction and was found to have omental involvement.
Ogunc et al24 have recommended omentopexy and have reported a zero incidence of catheter dysfunction. Crabtee et al25 reported a 0.5% incidence of drainage dysfunction after laparoscopic insertion and omentopexy when necessary, compared with 12.5% using laparoscopic insertion alone.16,25 Omentopexy adds little time and morbidity; thus, if the patient has an excessively large or long omen-tum extending into the pelvis, the omentopexy is now routinely performed by the author.
One drawback to placing the tip deep in the pelvis is the possibility of pelvic pain, which Ash26 has described in the past. He attributed the pain to stretching or shearing of the peritoneum during instillation of the fluid. Our only patient who had debilitating abdominal pain during PD complained of severe pain during the outflow of fluid and mostly in the subcostal region. Therefore, we do not believe there is a contraindication to placing the curl catheter deep into the cul-de-sac.
Abdominal wall herniation is a fairly common complication in patients undergoing peritoneal dialysis, occurring in up to 12% of cases.4–6 It is problematic as it requires repair and disrupts peritoneal dialysis for several weeks. Most patients who develop a hernia while on PD have small or occult hernias that are not diagnosed preoperatively. Therefore, a thorough preoperative examination to evaluate for hernias is mandatory. Furthermore, laparos-copy facilitates intraoperative inspection of the internal inguinal ring and identification of an occult inguinal hernia. The hernia may then be repaired before initiation of PD. In 5% of patients undergoing the open insertion technique, a hernia may develop around the catheter.6 Our insertion technique avoids this by placing the catheter through the abdominal wall by way of the introducer and sheath.
An umbilical trocar-site hernia occurred in one of the first 5 patients in whom we utilized a 5-mm trocar but did not close the fascia. We now close all midline trocar sites ≥5mm. If there are no intraabdominal adhesions, we only use a supraumbilical 3mm trocar and a right mid abdominal 2-mm trocar, virtually eliminating the risk of a postoperative incisional hernia.
CONCLUSION
Laparoscopic PD catheter insertion using minilaparoscopic instruments and a Quinton percutaneous insertion kit is safe, reproducible, and effective. It allows inspection of the abdominal cavity and adhesiolysis, omentectomy, or omentopexy when necessary. Furthermore, it facilitates exact placement of the catheter tip into the pelvis where it functions best. Utilization of this technique results in a low rate of PD catheter dysfunction.
Contributor Information
Stephen P. Haggerty, Department of Surgery, Evanston Northwestern Healthcare, Evanston, Illinois, USA..
Tallal M. Zeni, Department of Surgery, Evanston Northwestern Healthcare, Evanston, Illinois, USA..
Mike Carder, Evanston Northwestern Healthcare Highland Park Hospital, Highland Park, Illinois, USA..
Constantine T. Frantzides, Department of Surgery, Evanston Northwestern Healthcare, Evanston, Illinois, USA..
References:
- 1. Tenckhoff H, Schechter H. A bacteriologically safe perito-neal access device. Trans Am Soc Artif Intern Organs. 1968;14:181–187 [PubMed] [Google Scholar]
- 2. Nghiem DD. A technique of catheter insertion for uncomplicated peritoneal dialysis. Surg Gynecol Obstet. 1983;157:573–576 [PubMed] [Google Scholar]
- 3. Cronen PW, Moss JP, Simpson T, Rao M, Cowles L. Tenck-hoff catheter placement: surgical aspects. Am Surg. 1985;51(11):627–629 [PubMed] [Google Scholar]
- 4. Bullmaster JR, Miller SF, Finley RK, Jones LM. Surgical aspects of the Tenckhoff peritoneal dialysis catheter. A 7 year experience. Am J Surg. 1985;149:339–342 [DOI] [PubMed] [Google Scholar]
- 5. Olcott C, Feldman CA, Coplon NS, Oppenheimer ML, Mehigan JT. Continuous ambulatory peritoneal dialysis: Technique of catheter insertion and management of associated surgical complications. Am J Surg. 1983;146(1):98–102 [DOI] [PubMed] [Google Scholar]
- 6. Swartz R. Chronic peritoneal dialysis: mechanical and infectious complications. Nephron. 1985;40:29–37 [DOI] [PubMed] [Google Scholar]
- 7. Ash SR, Wolf GC, Bloch R. Placement of the Tenckhoff peritoneal dialysis catheter under peritoneoscopic visualization. Dialysis & Transplant. 1981;10:82–86 [Google Scholar]
- 8. Brandt CP, Franceschi D. Laparoscopic placement of perito-neal dialysis catheters in patients who have undergone prior abdominal operations. J Am Coll Surg. 1994;178(5):515–516 [PubMed] [Google Scholar]
- 9. Nijhuis PH, Smulders JF, Jakimowicz JJ. Laparoscopic introduction of a continuous ambulatory peritoneal dialysis (capd) catheter by a two-puncture technique. Surg Endosc. 1996;10:676–679 [DOI] [PubMed] [Google Scholar]
- 10. Watson DI, Paterson D, Bannister K. Secure placement of peritoneal dialysis catheters using a laparoscopic technique. Surg Laposc Endosc. 1996;6:35–37 [PubMed] [Google Scholar]
- 11. Crabtree JH, Fishman A. Videolaparoscopic implantation of long-term peritoneal dialysis catheters. Surg Endosc. 1999;13:186–190 [DOI] [PubMed] [Google Scholar]
- 12. Cala Z, Mimica Z, Ljutic D, Jankovic N, Varlaj-Knobloch V, Cala S. Laparoscopic placement of the peritoneal dialysis catheter using a specially designed trocar: a review of 84 patients. Dialysis & Transplantation 2000;29:722–727 [Google Scholar]
- 13. Barone G, Lightfoot M, Ketel B. Technique for laparoscopy-assisted complicated peritoneal dialysis catheter placement. J Laparoendosc Adv Surg Techn A. 2002;12:53–55 [DOI] [PubMed] [Google Scholar]
- 14. Lu CT, Watson DI, Elias TJ, Faull RJ, Clarkson AR, Bannister KM. Laparoscopic placement of peritoneal dialysis catheters: 7 years experience. Aust N Z J Surg. 2003;73:109–111 [DOI] [PubMed] [Google Scholar]
- 15. Manouras AJ, Kekis PB, Stamou KM, Konstadoulakis MM, Apostolidis NS. Laparoscopic placement of Oreopoulos-Zellerman catheters in CAPD patients. Peritoneal Dialysis Int. 2004;24:252–255 [PubMed] [Google Scholar]
- 16. Crabtree JH, Fishman A. A laparoscopic method for optimal peritoneal dialysis access. Am Surg. 2005;71:135–143 [DOI] [PubMed] [Google Scholar]
- 17. Harissis H, Katsios C, Koliousi E, et al. A new simplified one port laparoscopic technique of peritoneal dialysis catheter placement with intra-abdominal fixation. Am J Surg. 2006;192:125–129 [DOI] [PubMed] [Google Scholar]
- 18. Batey C, Crane J, Jenkins M, Johnston T, Munch L. Mini-laparoscopy-assisted placement of Tenckhoff catheters: an improved technique to facilitate peritoneal dialysis. J Endourol. 2002;16:681–684 [DOI] [PubMed] [Google Scholar]
- 19. Varela J, Elli E, Horgan S. Mini-laparoscopic placement of a peritoneal dialysis catheter. Surg Endosc. 2003;17(12):2025–2027 [DOI] [PubMed] [Google Scholar]
- 20. Yun E, Meng M, Brennan T, McAninch J, Santucci R, Rogers S. Novel microlaparoscopic technique for peritoneal dialysis catheter placement. Urology. 2003;61:1026–1028 [DOI] [PubMed] [Google Scholar]
- 21. Simopoulos C, Botaitis S, Polychronidis A, Tripsianis G, Karayiannakis A. Risk factory for cinversion of laparoscopic cholecystectomy to open cholecystectomy. Surg Endosc. 2005;19:905–909 [DOI] [PubMed] [Google Scholar]
- 22. Nelson H, et al. A comparison of laparoscopically assisted and open colectomy of colon cancer. N Eng J Med. 2004;350:2050–2059 [DOI] [PubMed] [Google Scholar]
- 23. Nicholson ML, Burton PR, Donnelly PK, Veitch PS, Walls J. The role of omentectomy in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis Int. 1991;11:330–332 [PubMed] [Google Scholar]
- 24. Ogunc G, Tuncer M, Ogunc D, Yardimsever M, Ersoy F. Laparoscopic omental fixation technique versus open surgical placement of peritoneal dialysis catheters. Surg Endosc. 2003;17:1749–1755 [DOI] [PubMed] [Google Scholar]
- 25. Crabtree JH, Fishman A. Selective performance of prophylactic omentopexy during laparoscopic implantation of perito-neal dialysis catheters. Surg Laparosc Endosc Percutan Tech. 2003;13:180–184 [DOI] [PubMed] [Google Scholar]
- 26. Ash SR, Carr DJ, Diaz-Buxo JA. Peritoneal access devices. Hydraulic function and biocompatibility. In: Nissenson AR, Fine RN, Gentile DE. eds. Clinical Dialysis. Norwalk, CT: Appleton & Lange; 1995;295–321 Vol. 11, No. 2 [Google Scholar]