Abstract
We present herein the case of a patient with solitary metachronous contralateral adrenal metastasis from renal cell cancer. The patient had undergone left radical nephrectomy and adrenalectomy for localized renal cancer 7 years previously. Laparoscopic transperitoneal right adrenalectomy was performed. The postoperative period was uneventful. Histology showed right adrenal metastasis from renal cancer. At 6-month follow-up, there was no evidence of recurrence.
Keywords: Laparoscopic adrenalectomy, Adrenal metastasis, Renal cancer
INTRODUCTION
Renal cell carcinoma (RCC) is the most common malignancy involving the kidney.1 Nearly 20% to 30% of patients with renal cell carcinoma have distant metastasis at presentation.2 Although RCC can metastasize to almost every organ, the most common metastatic sites are the lung parenchyma (50% to 60%),3 bone (30% to 40%),4 the liver (30% to 40%), and the brain (5%).5 Other rare sites of metastasis include the pancreas6,7 and parotid gland.8 Whereas metastasis of RCC to different sites is not uncommon, contralateral adrenal metastasis is very rare.
The average patient survival of metastatic RCC is about 4 months, and only 10% of these patients survive for 1 year. There is a small subset of patients where solitary metastasis is present either at the time of presentation or develops during follow-up after nephrectomy; these patients have a better survival.
Although adrenal metastases from RCC are rarely diagnosed in patients, these are not uncommonly found at autopsy.9,10 These are rare lesions that are diagnosed synchro-nously with the renal tumor. Most of the cases are in patients with multiple metastases. Only a few are detected metachronously.
Surgical removal of metastatic lesions is the only known effective treatment in patients with solitary metastasis, with 14% to 38% surviving 5 years or more.11–13 Several investigators have re-ported better survival for metastatic RCC in patients who have a solitary metastasis that appears more than 18 months after nephrectomy.11,12,14
We present herein a case of large, metachronous, contralateral adrenal metastasis from primary left renal cell adenocarcinoma that was removed completely by the laparoscopic approach. The patient had left nephrectomy and left adrenalectomy 7 years earlier.
CASE REPORT
A 44-year-old Caucasian man underwent left radical nephrectomy, including ipsilateral adrenalectomy in 1999, for a left renal mass and did not receive adjuvant chemo-therapy. The histology report showed adenocarcinoma.
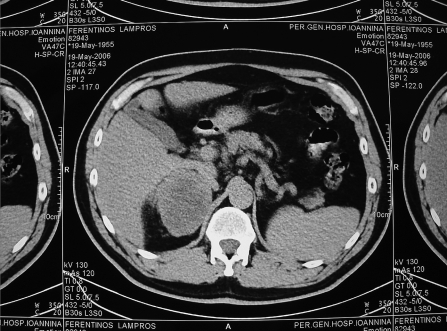
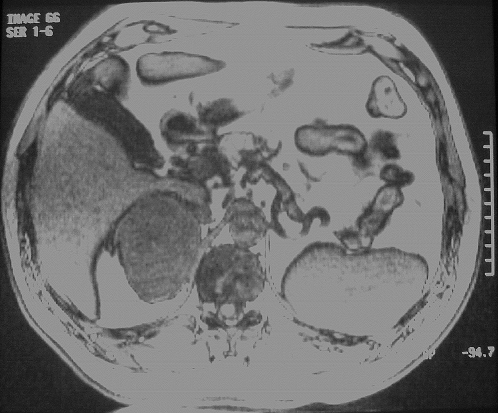
The patient did well until 7 years later, when an annual follow-up abdominal computerized tomography (CT) scan revealed a 9×8-cm hypervascular mass in the right adrenal (Figure 1). Magnetic resonance imaging (MRI) confirmed a malignant-appearing mass measuring 9×8cm arising from the right adrenal. After intravenous injection of gadolinium, the mass showed a hypervascular mass (Figure 2). No evidence was found of calcification or invasion of adjacent tissues or organs. No other abdominal abnormalities were noted.
Figure 1.
Abdominal computed tomographic scan suggested possible metastatic tumor to the right adrenal gland.
Figure 2.
Abdominal magnetic resonance image showing the right adrenal mass.
The patient was in good health and asymptomatic. Blood pressure was within the normal range. At physical examination, no abnormalities were found. Radiography of the thorax and an electrocardiogram were normal. Routine blood and urine tests as well as tumor markers showed no abnormality. On metabolic evaluation, 24-hour urine collection for 17-ketosteroids, 17-hydroxycorticoids, metanephrines, cortisol, and vanillylmandelic acid were within normal limits.
Surgical Technique
The laparoscopic approach was chosen. We prefer a transperitoneal lateral decubitus approach as the best for maximal exposure of the gland and major vessels. Using the Hasson technique, a 12-mm trocar, one 10-mm trocar, one 12-mm, and one 5-mm trocar were inserted below the costal margin. Laparoscopic exploration of the abdominal cavity revealed no abnormalities, and the tumor was easily identified. The right triangular ligament and the retroperitoneal liver attachments were cauterized and divided to allow liver retraction. After dividing the retroperitoneum, the inferior vena cava (IVC) was identified. The inferior periadrenal fat was carefully dissected from the upper pole of the right kidney and the renal vein identified. The right adrenal vein was subsequently identified, dissected, double-clipped, and divided. The inferior and superior adrenal vessels were cauterized with ultrasonic scissors following division of the right adrenal vein. The entire specimen was placed intact into a laparoscopy entrapment sack and extracted through an extension of the incision done for the Hasson technique.
The pneumoperitoneum was aspirated through ports before specimen extraction. The port-site wounds were irrigated with a solution of povidone iodine 10% followed by normal saline. The fascia of the 3 trocar sites was closed with a 1-0 Prolene stitch. The procedure lasted 3 hours, the estimated intraoperative blood loss was 600 mL, and the patient was not transfused. The surgical specimen measured 9×8 cm and weighed 330 g.
The patient was started on immediate steroid replacement, with the hydrocortisone dosage tapered to 20mg 3 times daily, in addition to 0.1 mg fludrocortisone daily.
The postoperative period was uneventful, and the patient was discharged on the second postoperative day with bowel function returned to normal and the patient able to return to normal physical activity.
Histology confirmed a metastatic tumor from renal aden-ocarcinoma.
Six months after the procedure, there was no evidence of recurrence on abdominal CT scan, and the cosmetic result was satisfactory.
DISCUSSION
RCC metastasis may be found synchronously with the primary tumor or in various organs many years after the primary lesion has been treated. Metastasis of RCC to the contralateral adrenal gland is very rare. In reviews of the literature, the majority of the cases described involved synchronous metastasis.15–17 Most metachronous metastases are identified in the first or second year after nephrectomy, and 25% of patients develop metastatic disease within 5 years of nephrectomy.18 Metastasis has been diagnosed as late as 23 years after nephrectomy.19,20
Adrenal metastasis is rarely diagnosed during life. In fact, clinical signs and symptoms are rare in these patients. However, recently improvements in noninvasive radiological techniques, such as computerized tomography (CT), selective arteriography, and magnetic resonance imaging (MRI), have improved the clinical detection of metastatic lesions. CT-guided needle biopsy is currently available in some centers, including ours. Biopsy of adrenal masses may be helpful in doubtful cases, although in general the diagnosis has been problematic. However, several concerns have been raised, among which are that the capsule of an adrenal tumor is thin and the lesion tends to be brittle. As a result, an intrinsic risk exists of hemorrhage.21,22
In some cases, failure to use routine imaging studies might explain the delayed detection. However, a more possible explanation for the delayed diagnosis of metastasis is that some metastases can be very slow growing.21
RCC is known for its varied presentation and its propensity to metastasize by way of both venous and lymphatic routes. Conversely, it is believed that hematogenous metastasis to the adrenal gland is more common than lymphogenous extension due to the rich blood supply of the adrenal gland and its high blood volume-to-unit weight ratio.23
The prognosis for patients with untreated widely metastatic renal cell carcinoma is dismal. Nevertheless, in patients with solitary or limited metastases, removal of the RCC metastasis is associated with prolonged survival. Studies have shown significant differences in survival between synchronous and metachronous solitary metastases, the synchronous group being the worst.12,14,24 Many studies have reported that patients with adrenal metastasis occurring a long time after nephrectomy had a better prognosis than those with a short interval to diagnosis. Several investigators have noted better survival for metastatic RCC in patients who have a solitary metastasis that appears more than 18 months after nephrectomy.11,12,14,25,26 In other studies, a better prognosis has been reported in patients with a disease-free interval of more than 12 months.27 Furthermore for some investigators, the disease-free interval required for better survival is only 6 months. The observation of poorer survival most likely is an indicator of tumor biology.
After metastasectomy, overall median survival ranges from 26 months to 45 months. The 3-year survival rate ranges from 35% to 60% and 5-year survival ranges from 14% to 38%.11,16,17
A broad range of treatments, including chemotherapy, hormonal therapy, and radiotherapy, have failed to markedly improve survival. Moreover, despite the early promising results with immunotherapy, a complete response occurs in less than 15%. Therefore, aggressive treatment of such lesions is indicated. Complete extirpation of all tumor clearly is important.17,19
Laparoscopic adrenalectomy has been performed successfully transperitoneally and retroperitoneally to treat a variety of benign adrenal pathological conditions.
Laparoscopic procedures are associated with less postoperative discomfort, decreased hospital stay, less postoperative disability, and a lower rate of complications. Few absolute contraindications exist for laparoscopic adrenalectomy, and most of them are not specific to adrenal surgery. Large but well-encapsulated adrenal masses without evidence of local invasion can be removed laparoscopically.28 The lateral transabdominal approach offers the best visualization of major vessels adjacent to the adrenals.29
The role of laparoscopic surgery for malignant adrenal tumors is controversial, because few data exist in the literature for this rare disease.
Concerns have focused on the possibility of tumor rupture due to manipulation with laparoscopic instruments with the attendant fear of disseminating malignant cells inside the peritoneal cavity.
Three cases of diffuse peritoneal dissemination and death of patients who underwent laparoscopic adrenalectomy for adrenal cancer have been reported.30 Moreover in a larger study, 13 patients, 6 with adrenal cortical carcinoma and 7 with metastasis, were reported. The mean size of the malignant lesions was 5.9cm. The mean follow-up was 30 months, during which 3 patients died, 1 from endoperitoneal and trocar port-site seeding.31 In another study, 31 patients underwent 33 laparoscopic adrenalectomies. In 26 cases, metastatic cancer and primary adrenal malignancy were present in 7. Follow-up was 26 months, during which 15 patients died. Local recurrence was noted in 7 patients. However, no port-site metastases occurred. The 5-year survival reached 40%.32
On the contrary, Heniford and colleagues in a review of 10 patients with metastatic adrenal tumors and 1 patient with adrenocortical carcinoma reported no local or port-site recurrence at a mean follow-up of 8.3 months.33 Furthermore, there is another study of 8 patients with malignancies, 4 with cortical carcinomas and 4 with metastases (from renal carcinoma, breast carcinoma, leiomyosarcoma and rhabdoid sarcoma). Mean follow-up was 40.5 months. During follow-up, 3 patients with cortical carcinomas were disease-free and 1 died of a stroke, 2 had metastases (1 leiomyosarcoma and 1 breast carcinoma) 1 died of disease, and 2 were disease free. No port-site metastases occurred.34 In the largest series reported in the literature,35 21 patients who underwent laparoscopic adrenalectomy for malignant tumors were reviewed. Three locoregional recurrences occurred 1 to 2.5 years after resection (2 local and 1 lymph node metastasis) in the 6 patients with primary adrenal cancer. There were no local recurrences in the 13 patients with metastatic adrenal tumors.
A study has focused on solitary adrenal gland metastasis in patients operated on for nonsmall cell lung cancer. Eleven patients had solitary adrenal metastases. All of them underwent laparoscopic adrenalectomy. Three were still alive and well at 37 months to 80 months after the lung resection. One patient (who underwent bilateral adrenal-ectomy) was still alive at 44 months with local relapse. Two patients died 5 and 6 months after the adrenalectomy of other causes; 1 died at 14 months for local and systemic relapse, and the remaining 3 patients died at 12 to 38 months for systemic relapse.36
CONCLUSION
We report a case of metachronous, contralateral metastasis from left renal adenocarcinoma, occurring 7 years after curative radical nephrectomy, including left adrenalectomy. Despite the large size of the tumor, laparoscopic adrenalectomy was successfully performed. Laparoscopic adrenalectomy resulted in a short hospital stay, minimal blood loss, and minimal postoperative analgesic requirement. There was no other evidence of metastatic disease at adrenalectomy, or at 6-month follow-up.
Patients having metachronous, contralateral adrenal metastasis from renal cell carcinoma benefit from the surgical resection of the lesion. Patients with a disease-free interval of more than 1 year before development of solitary metastasis appear to have better survival. The laparoscopic approach, having more advantages versus open surgery, is feasible but necessitates experience in advanced laparoscopic surgery. The benefits to the patient of earlier ambulation, decreased pain, better cosmetic results, and lower percentage of incisional hernia must be weighed against the as yet unknown long-term results. Extensive experience in advanced laparoscopic techniques and open adrenal surgery are mandatory to manipulate and excise laparoscopically malignant tumors.
Contributor Information
G. N. Zografos, Third Department of Surgery, Athens General Hospital , “G. Gennimatas”, Greece.
A. Farfaras, Third Department of Surgery, Athens General Hospital , “G. Gennimatas”, Greece.
C. Aggeli, Third Department of Surgery, Athens General Hospital , “G. Gennimatas”, Greece.
G. Kontogeorgos, Department of Histopathology, Athens General Hospital, Greece.
M. Pagoni, Department of Internal Medicine, Athens General Hospital, Greece.
S. Vogiati, Department of Internal Medicine, Athens General Hospital, Greece.
G. Vasiliadis, Third Department of Surgery, Athens General Hospital , “G. Gennimatas”, Greece.
G. Papastratis, Third Department of Surgery, Athens General Hospital , “G. Gennimatas”, Greece.
References:
- 1. Paulson DF. The urinary system. In: Sabiston DC , ed. Textbook of surgery. The biological basis of modern surgical practice. Philadelphia, PA: WB Saunders Company; 1997 [Google Scholar]
- 2. Matveev VB, Gurarii LL, Began-Bogatskii KM. Surgical treatment of late metastases of kidney cancer. Urol Nefrol (Mosk). 1999;2:51–52 [PubMed] [Google Scholar]
- 3. Cozzoli A, Milano S, Cancarini G, Zanotelli T, Cosciani Cunio S. Surgery of lung metastases in renal cell carcinoma. Br J Urol. 1995;75:445–447 [DOI] [PubMed] [Google Scholar]
- 4. Kollender Y, Bickels J, Price WM, et al. Metastatic renal cell carcinoma of bone: indications and technique of surgical intervention. J Urol. 2000;164:1505–1508 [PubMed] [Google Scholar]
- 5. Ritchie AWS, Chisholm GD. The natural history of renal carcinoma. Semin Oncol. 1983;10:390–400 [PubMed] [Google Scholar]
- 6. Andoh H, Kurokawa T, Yasui O, Shibata S, Sato T. Resection of a solitary pancreatic metastasis from renal cell carcinoma with a gallbladder carcinoma: report of a case. Surg Today. 2004;34:272–275 [DOI] [PubMed] [Google Scholar]
- 7. Kassabian A, Stein J, Jabbour N. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211–215 [DOI] [PubMed] [Google Scholar]
- 8. Park YW, Hlivko TJ. Parotid gland metastasis from renal cell carcinoma. Laryngoscope. 2002;112:453–456 [DOI] [PubMed] [Google Scholar]
- 9. Hajdu SI, Thomas AG. Renal cell carcinoma at autopsy. J Urol. 1967;97:978–982 [DOI] [PubMed] [Google Scholar]
- 10. Saitoh H, Nakayama M, Nakamura K, Satoh T. Distant metastasis of renal adenocarcinoma in nephrectomized cases. J Urol. 1982;127:1092–1095 [DOI] [PubMed] [Google Scholar]
- 11. Kessler O, Mukamel E, Weinstein R, Gayer E, Konichezky M. Metachronous renal cell carcinoma metastasis to the contralateral adrenal gland. Urology. 1998;51(4):539–543 [DOI] [PubMed] [Google Scholar]
- 12. Tolia BM, Whitmore WF. Solitary metastasis from renal cell carcinoma. J Urol. 1975;144:836–838 [DOI] [PubMed] [Google Scholar]
- 13. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and treatment of renal cell carcinoma. A clinical and pathological study of 309 cases. Cancer. 1971;28:1165–1177 [DOI] [PubMed] [Google Scholar]
- 14. O'Dea MJ, Zincke H, Utz DC, Bernatz PE. The treatment of renal cell carcinoma with solitary metastasis. J Urol. 1978;120:540–542 [DOI] [PubMed] [Google Scholar]
- 15. Huisman TK, Sands JP. Renal cell carcinoma with solitary metachronous contralateral adrenal metastasis. Urology. 1991; 38(4):364–368 [DOI] [PubMed] [Google Scholar]
- 16. Elashry O, Clayman R, Soble J, McDougall E. Laparoscopic adrenalectomy for solitary matachronous contralateral adrenal metastasis from renal cell carcinoma. J Urol. 1997;157:1217–1222 [PubMed] [Google Scholar]
- 17. Kim SH, Brennan MF, Russo P, Burt ME, Coit DG. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer. 1998;82:389–394 [PubMed] [Google Scholar]
- 18. Ritchie AWS, deKernion JB. The natural history and clinical features of renal carcinoma. Semin Nephrol. 1987;7:131–139 [PubMed] [Google Scholar]
- 19. Thyavihally YB, Mahantshetty U, Chamarajanagar RS, Raibhattanavar SG, Tongaonkar HB. Management of renal cell carcinoma with solitary metastasis. World J Surg Oncol. 2005;3:48–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mesurolle B, Mignon F, Travagli JP, Meingan P, Vanel D. Late presentation of solitary contralateral adrenal metastasis of renal cell carcinoma. Eur Radiol. 1997;7:557–558 [DOI] [PubMed] [Google Scholar]
- 21. Lau WX, Zinke CM, Johse CM, Chevillet JC, Weaver AJ, Blute MI. Contralateral adrenal metastasis of renal cell carcinoma: treatment, outcome and a review. Br J Urol. 2003;91:775–779 [DOI] [PubMed] [Google Scholar]
- 22. Dechet CB, Zincke H, Sebo TJ, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol. 2003;169:71–74 [DOI] [PubMed] [Google Scholar]
- 23. Campbell CNI, Middleton RG, Rigby OF. Adrenal metastasis in renal cell carcinoma. Urology. 1983;21:403. [DOI] [PubMed] [Google Scholar]
- 24. Rafla S. Renal cell carcinoma: natural history and results of treatment. Cancer. 1970;25:26–40 [DOI] [PubMed] [Google Scholar]
- 25. Talley RV, Moorhead EL, 2nd, Tucker WG, San Diego EL, Brennan MJ. Treatment of metastatic hypernephroma. JAMA. 1969;207:322–328 [PubMed] [Google Scholar]
- 26. Skinner DG, Vermillion CD, Golvin KB. The surgical management of renal cell carcinoma. J Urol. 1972;107:705–708 [DOI] [PubMed] [Google Scholar]
- 27. Tongaonkar HB, Kulkarni JN, Kamat MR. Solitary metastases from renal cell carcinoma: A review. J Surg Oncol. 1992;49:45–48 [DOI] [PubMed] [Google Scholar]
- 28. Brunt ML, Moley JF. Adrenal incidentaloma. World J Surg. 2001;25:905–913 [DOI] [PubMed] [Google Scholar]
- 29. Zografos GN, Markou A, Ageli C, et al. Laparoscopic surgery for adrenal tumors. A retrospective analysis. Hormones. 2006;5(1):52–56 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki K, Ushiyama T, Mugiya S, et al. Hazards of laparoscopic adrenalectomy in patients with adrenal malignancy. J Urol. 1997;158:2227. [DOI] [PubMed] [Google Scholar]
- 31. Porpiglia F, Fiori C, Tarabuzzi R, et al. Is laparoscopic adrenalectomy feasible for adrenocortical carcinoma or metastasis? BJU Int. 2004;94(7):1026–1029 [DOI] [PubMed] [Google Scholar]
- 32. Moinzadeh A, Gill IS. Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol. 2005;173(2):519–525 [DOI] [PubMed] [Google Scholar]
- 33. Heniford BT, Arca MJ, Walsh RM, GilI IS. Laparoscopic adrenalectomy for cancer. Semin Surg Oncol. 1999;16:293–306 [DOI] [PubMed] [Google Scholar]
- 34. Giraudo G, Del Genio G, Porpiglia F, Parini D, Garrone C, Morino M. Laparoscopic adrenalectomy in multiple endocrine tumors, in secreting and non-secreting lesions. Minerva Chir. 2004;59(1):1–5 [PubMed] [Google Scholar]
- 35. Kebebew E, Siperstein A, Clark O, Quan-Yang D. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal tumors. Arch Surg. 2002;137:948–953 [DOI] [PubMed] [Google Scholar]
- 36. Lucchi M, Dini P, Ambrogi MC, et al. Metachronous adrenal masses in resected non-small cell lung cancer patients: therapeutic implications of laparoscopic adrenalectomy. Eur J Cardiothorac Surg. 2005;27(5):753–756 [DOI] [PubMed] [Google Scholar]