Abstract
Palmitate, glucose, and glycerol oxidation to CO2 have been investigated in the fasted state in ten normal subjects and nine patients (six hyperlipoproteinemias, one xanthomatosis, and two glycogenosis) after intravenous injection of [1-14C]palmitate, [1-14C]glucose, or [1-14C]glycerol in tracer amounts. The specific activities and concentrations of plasma palmitate, glycerol, or glucose and expired CO2 were measured at various intervals after the injection for a period of 24 h. All the studies were analyzed in terms of a multicompartment model describing the structure for each of the subsystems, the transfer of carbon label between subsystems, and the oxidation to CO2. A bicarbonate subsystem was also included in the model to account for its role in shaping the CO2 curves.
All the CO2 activity following a palmitate injection could be accounted for by a direct oxidative pathway from plasm FFA with the addition of a 20-min delay compartment. The same also applied to glucose, except that the delay compartment had a mean time of about 150 min. Only about a third of the injected glycerol was directly oxidized to CO2 from plasma; the delay time was about 4 min. Most of the remainder was converted to glucose.
In normals about 45% of the FFA is oxidized to CO2 directly. This constitutes about 30% of the total CO2 output. In hyperlipemia the CO2 output is nearly unchanged and the contribution from FFA is nearly the same. There is a considerable increase (factor of 2), however, in FFA mobilization, most of which is probably diverted to triglyceride synthesis.
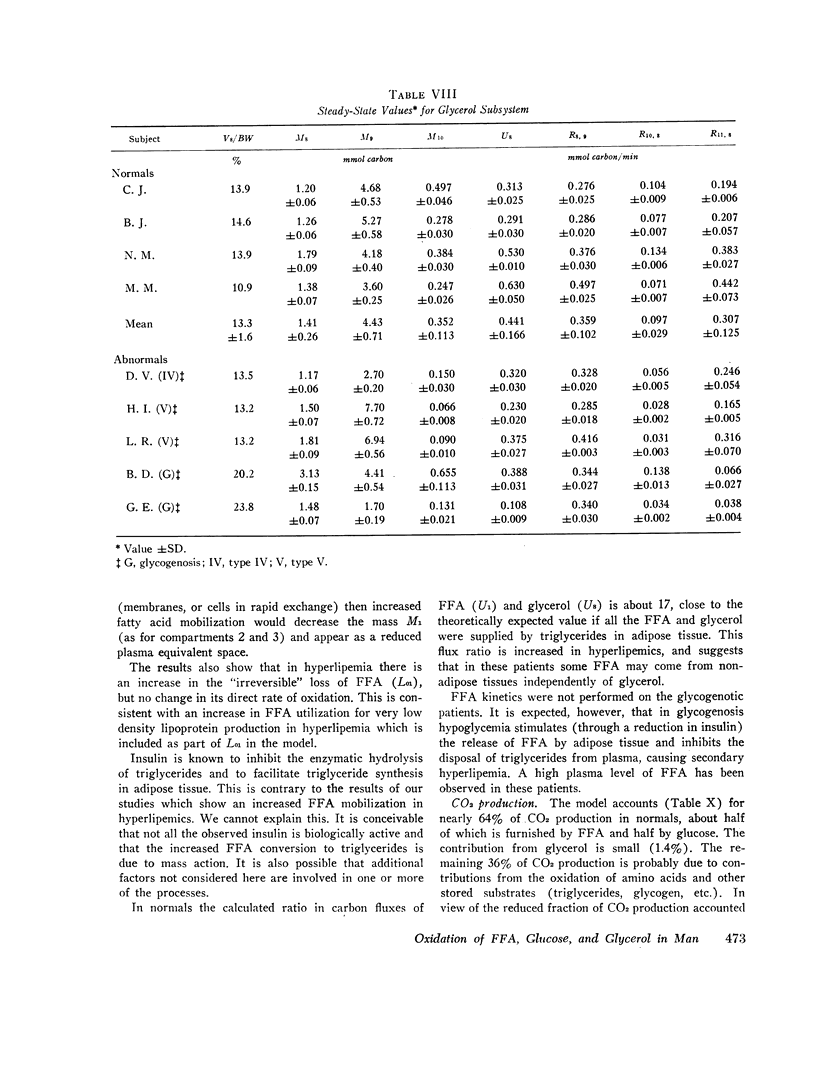
The glucose and glycerol subsystems are roughly the same in normals and hyperlipemics. About 50% of glucose is oxidized by the direct pathways which accounts for about 35% of the CO2 output. Glycerol accounts for only 1.5% of the CO2 produced.
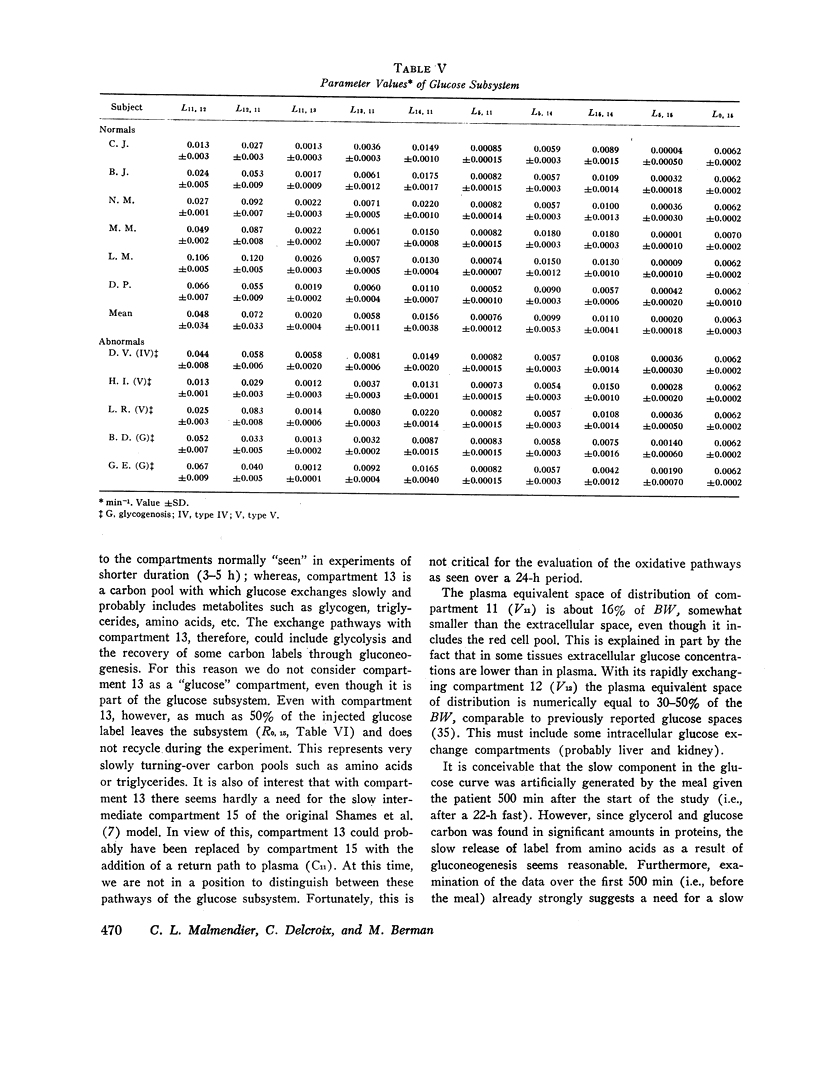
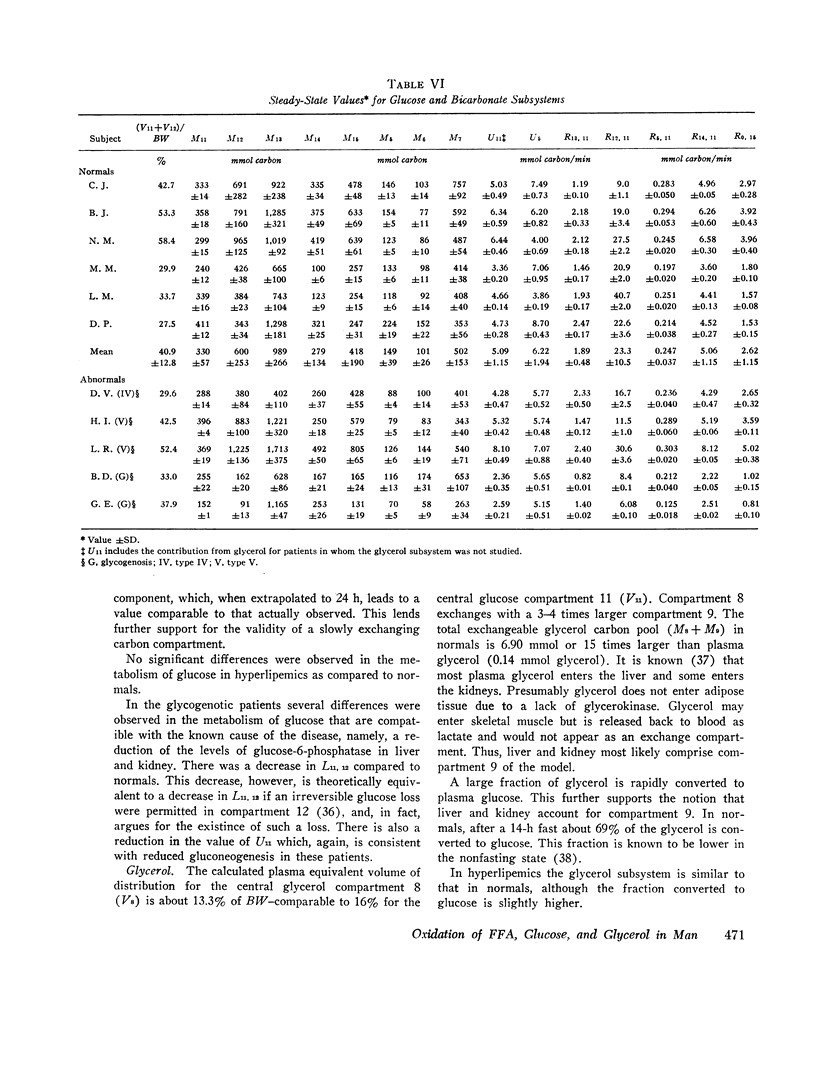
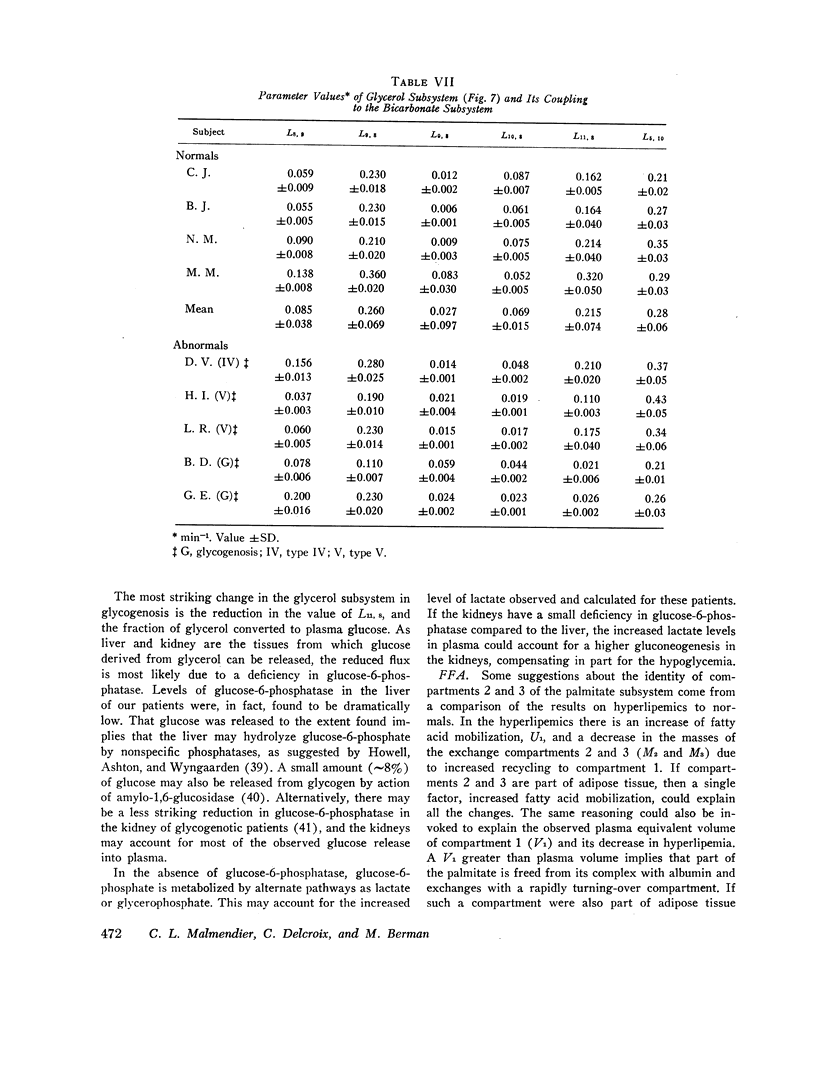
Major changes occurred in the glycerol and glucose subsystems in glycogenosis. The changes are consistent with the known deficiency in glucose-6-phosphatase in this disorder. There is a considerable reduction (factor of 2 or more) in the release of glucose to plasma (gluconeogenesis) and in the conversion of glycerol to glucose.
Despite the integration of the kinetics of the glucose, glycerol, and FFA subsystems over a 24-h period, 36% of the CO2 production was still unaccounted for in normals and 50% in hyperlipemics. Thus, some of the carbon must wind up in very slowly turning-over pools which supply CO2 through subsystems not covered in these studies (triglycerides, glycogen, amino acids, etc.).
All the modeling was carried out with the aid of the SAAM25 computer program.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER N., SHREEVE W. W., SHIPLEY R. A., INCEFY G. E., MILLER M. C14 studies in carbohydrate metabolism. I. The oxidation of glucose in normal human subjects. J Biol Chem. 1954 Dec;211(2):575–592. [PubMed] [Google Scholar]
- BERNSTEIN I. A., LENTZ K., MALM M., SCHAMBYE P., WOOD H. G. Degradation of glucose-C14 with Leuconostoc mesenteroides; alternate pathways and tracer patterns. J Biol Chem. 1955 Jul;215(1):137–152. [PubMed] [Google Scholar]
- BORCHGREVINK C. F., HAVEL R. J. TRANSPORT OF GLYCEROL IN HUMAN BLOOD. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:946–949. doi: 10.3181/00379727-113-28539. [DOI] [PubMed] [Google Scholar]
- BOY J. Automatic determination of blood cholesterol. J Clin Pathol. 1963 Mar;16:178–180. doi: 10.1136/jcp.16.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGDON J. H. Colorimetric determination of blood lipides. J Biol Chem. 1951 Jun;190(2):513–517. [PubMed] [Google Scholar]
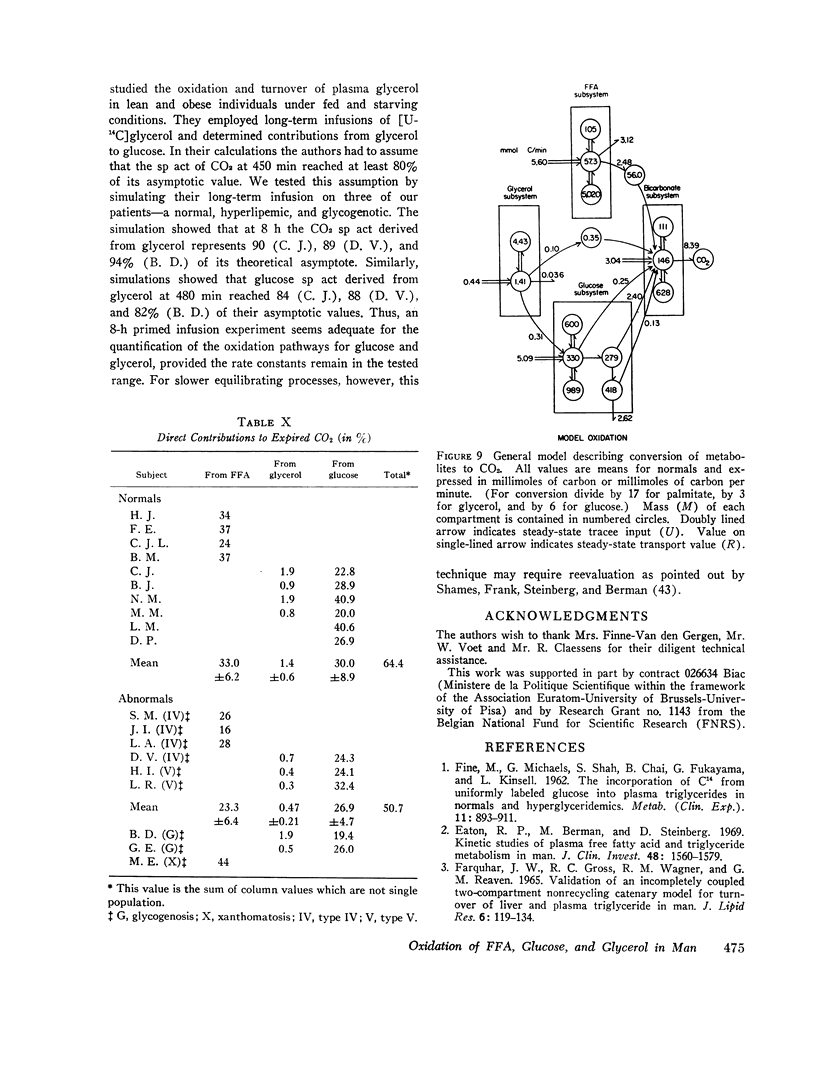
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell G. L., Berman M., Robertson J. S. Nomenclature for tracer kinetics. Int J Appl Radiat Isot. 1968 Mar;19(3):249–262. doi: 10.1016/0020-708x(68)90022-7. [DOI] [PubMed] [Google Scholar]
- CARLSON L. A., WADSTROM L. B. Determination of glycerides in blood serum. Clin Chim Acta. 1959 Mar;4(2):197–205. doi: 10.1016/0009-8981(59)90130-5. [DOI] [PubMed] [Google Scholar]
- COXON R. V., ROBINSON R. J. The transport of radioactive carbon dioxide in the blood stream of the dog after administration of radioactive bicarbonate. J Physiol. 1959 Oct;147:469–486. doi: 10.1113/jphysiol.1959.sp006257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. P., Berman M., Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969 Aug;48(8):1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR J. W., GROSS R. C., WAGNER R. M., REAVEN G. M. VALIDATION OF AN INCOMPLETELY COUPLED TWO-COMPARTMENT NONRECYCLING CATENARY MODEL FOR TURNOVER OF LIVER AND PLASMA TRIGLYCERIDE IN MAN. J Lipid Res. 1965 Jan;6:119–134. [PubMed] [Google Scholar]
- FINE M., MICHAELS G., SHAH S., CHAI B., FUKAYAMA G., KINSELL L. The incorporation of C14 from uniformly labeled glucose into plasma triglycerides in normals and hyperglyceridemics. Metabolism. 1962 Aug;11:893–911. [PubMed] [Google Scholar]
- FREDRICKSON D. S., GORDON R. S., Jr The metabolism of albumin-bound C14-labeled unesterified fatty acids in normal human subjects. J Clin Invest. 1958 Nov;37(11):1504–1515. doi: 10.1172/JCI103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKSON D. S., ONO K., DAVIS L. L. LIPOLYTIC ACTIVITY OF POST-HEPARIN PLASMA IN HYPERGLYCERIDEMIA. J Lipid Res. 1963 Jan;4:24–33. [PubMed] [Google Scholar]
- Field R. A. The glycogenoses: von Gierke's disease, acid maltase deficiency, and liver glycogen phosphorylase deficiency. Am J Clin Pathol. 1968 Jul;50(1):20–28. doi: 10.1093/ajcp/50.1.20. [DOI] [PubMed] [Google Scholar]
- GAITONDE M. K., MARCHI S. A., RICHTER D. THE UTILIZATION OF GLUCOSE IN THE BRAIN AND OTHER ORGANS OF THE CAT. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:124–136. doi: 10.1098/rspb.1964.0031. [DOI] [PubMed] [Google Scholar]
- HARRIS R. C., OLMO C. Liver and kidney glucose-6-phosphatase activity in children with normal and diseased organs. J Clin Invest. 1954 Sep;33(9):1204–1209. doi: 10.1172/JCI102994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL R. R., ASHTON D. M., WYNGAARDEN J. B. Glucose-6-phosphatase deficiency glycogen storage disease. Studies on the interrelationships of carbohydrate, lipid, and purine abnormalities. Pediatrics. 1962 Apr;29:553–565. [PubMed] [Google Scholar]
- Havel R. J., Ekelund L. G., Holmgren A. Kinetic analysis of the oxidation of palmitate-1-14C in man during prolonged heavy muscular exercise. J Lipid Res. 1967 Jul;8(4):366–373. [PubMed] [Google Scholar]
- REICHARD G. A., Jr, JACOBS A. G., KIMBEL P., HOCHELLA N. J., WEINHOUSE S. Blood glucose replacement rates in normal and diabetic humans. J Appl Physiol. 1961 Sep;16:789–795. doi: 10.1152/jappl.1961.16.5.789. [DOI] [PubMed] [Google Scholar]
- SEGAL S., BERMAN M., BLAIR A. The metabolism of variously C14-labeled glucose in man and an estimation of the extent of glucose metabolism by the hexose monophosphate pathway. J Clin Invest. 1961 Jul;40:1263–1279. doi: 10.1172/JCI104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIPLEY R. A., BAKER N., INCEFY G. E., CLARK R. E. C14 studies in carbohydrate metabolism. IV. Characteristics of bicarbonate pool system in the rat. Am J Physiol. 1959 Jul;197(1):41–46. doi: 10.1152/ajplegacy.1959.197.1.41. [DOI] [PubMed] [Google Scholar]
- SHREEVE W. W., HENNES A. R., SCHWARTZ R. Production of C14O2 from 1- and 2-C14-acetate by human subjects in various metabolic states. Metabolism. 1959 Sep;8:741–756. [PubMed] [Google Scholar]
- Schimmel R. J., Knobil E. Insulin, free fatty acids, and stimulation of hepatic gluconeogenesis during fasting. Am J Physiol. 1970 Jun;218(6):1540–1547. doi: 10.1152/ajplegacy.1970.218.6.1540. [DOI] [PubMed] [Google Scholar]
- Shames D. M., Berman M., Segal S. Effects of thyroid disease on glucose oxidative metabolism in man. A compartmental model analysis. J Clin Invest. 1971 Mar;50(3):627–641. doi: 10.1172/JCI106533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames D. M., Frank A., Steinberg D., Berman M. Transport of plasma free fatty acids and triglycerides in man: a theoretical analysis. J Clin Invest. 1970 Dec;49(12):2298–2314. doi: 10.1172/JCI106449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeve W. W. Effects of insulin on the turnover of plasma carbohydrates and lipids. Am J Med. 1966 May;40(5):724–734. doi: 10.1016/0002-9343(66)90153-7. [DOI] [PubMed] [Google Scholar]
- Stewart C. P., Hendry E. B. The phospholipins of blood. Biochem J. 1935 Jul;29(7):1683–1689. doi: 10.1042/bj0291683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEDA Y., REEVE E. B. Studies of the metabolism and distribution of albumin with autologous I131-albumin in healthy men. J Lab Clin Med. 1963 Feb;61:183–202. [PubMed] [Google Scholar]
- WIELAND O. Eine enzymatische Methode zur Bestimmung von Glycerin. Biochem Z. 1957;329(4):313–319. [PubMed] [Google Scholar]
- Waterhouse C., Baker N., Rostami H. Effect of glucose ingestion on the metabolism of free fatty acids in human subjects. J Lipid Res. 1969 Sep;10(5):487–494. [PubMed] [Google Scholar]
- Winkler B., Rathgeb I., Steele R., Altszuler N. Conversion of glycerol to glucose in the normal dog. Am J Physiol. 1970 Aug;219(2):497–502. doi: 10.1152/ajplegacy.1970.219.2.497. [DOI] [PubMed] [Google Scholar]


