Abstract
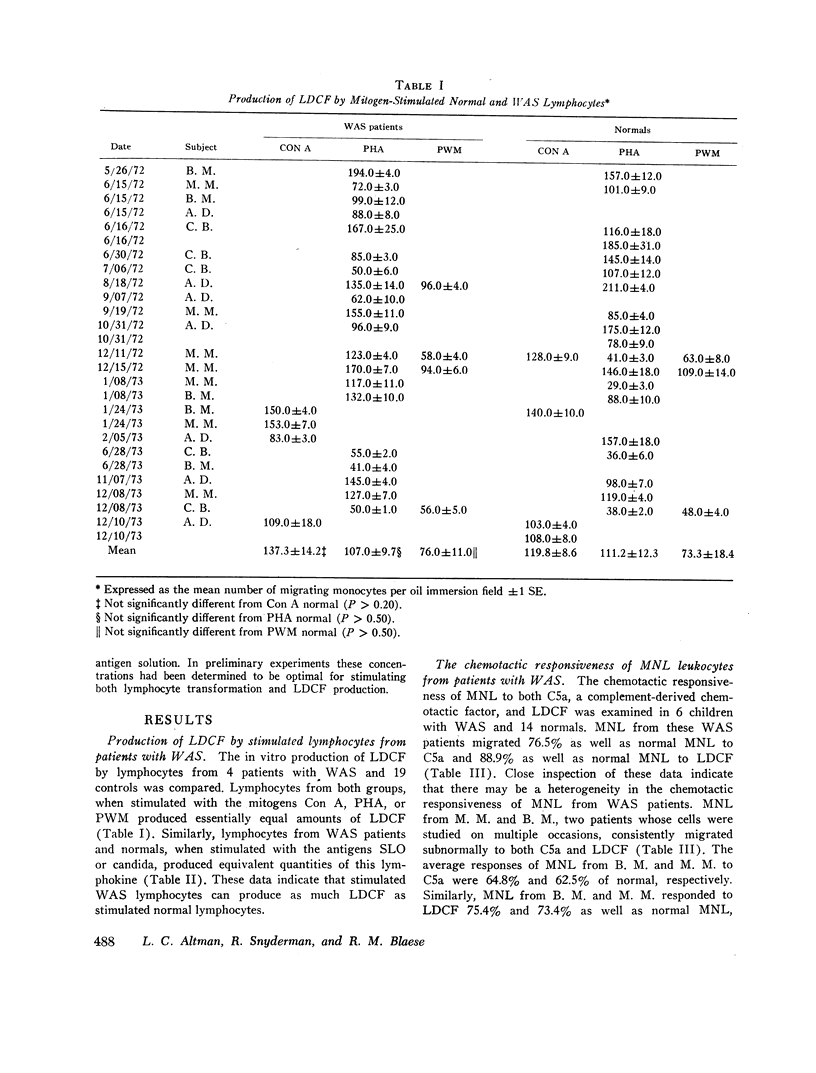
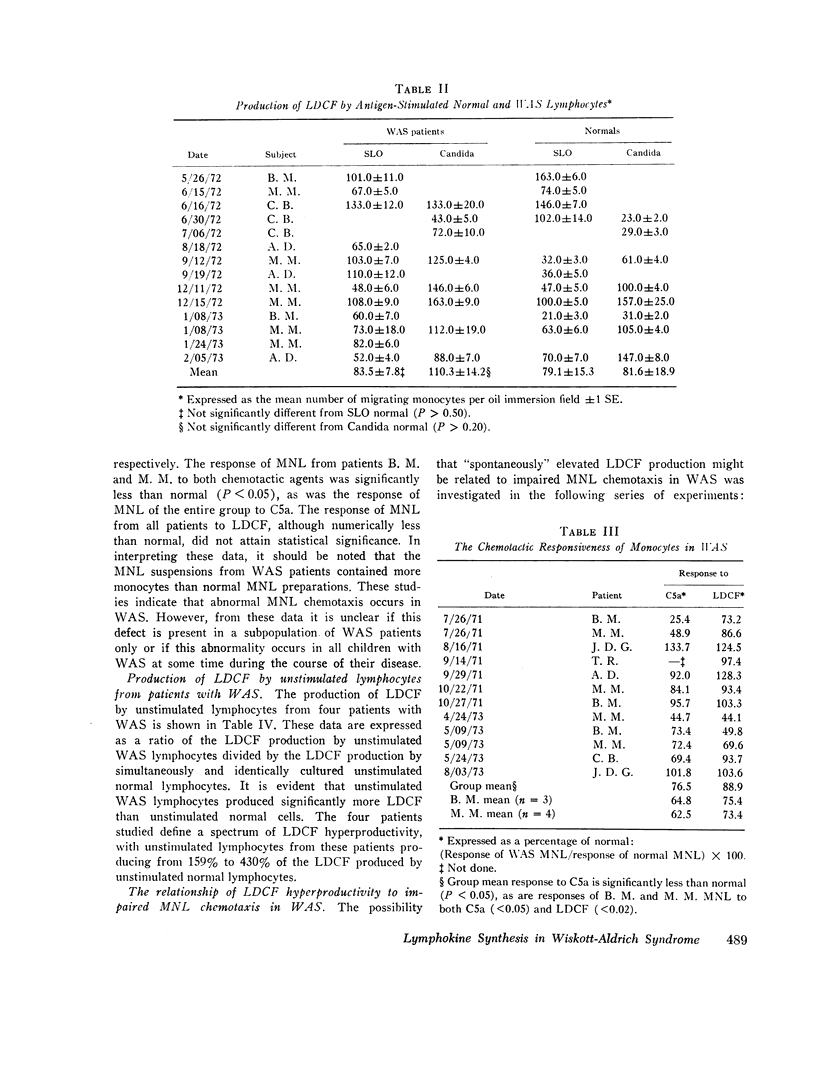
Wiskott-Aldrich syndrome is characterized by numerous humoral and cellular immune abnormalities including anergy, defective antibody production, and increased immunoglobulin synthesis. To define better the mechanisms of defective cellular immunity in this disorder, lymphoproliferative responses, lymphokine production, and the chemotactic responsiveness of mononuclear leukocytes (MNL) from patients with Wiskott-Aldrich syndrome were quantitated. Peripheral blood lymphocytes from these patients produced normal amounts of a lymphocyte-derived chemotactic factor (LDCF); however, their lymphoproliferative responses were frequently depressed, particularly to antigenic stimuli. In the absence of exogenous antigens or mitogens, lymphocytes from patients with Wiskott-Aldrich syndrome produced significantly more LDCF than unstimulated normal lymphocytes. In fact, this unstimulated LDCF production frequently approached the level produced by normal cells only after antigen or mitogen stimulation.
The chemotactic responsiveness of MNL from Wiskott-Aldrich syndrome patients was impaired, particularly in those patients with the highest rates of unstimulated LDCF production. Furthermore, normal MNL chemotactic responsiveness could be impaired by preincubation of these cells in either LDCF or plasma from Wiskott-Aldrich syndrome patients. These observations suggest that the regulation of LDCF synthesis is abnormal in Wiskott-Aldrich syndrome, and that a humoral chemotactic inhibitor, perhaps LDCF, “deactivates” the circulating MNL of patients with this syndrome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRICH R. A., STEINBERG A. G., CAMPBELL D. C. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954 Feb;13(2):133–139. [PubMed] [Google Scholar]
- Altman L. C., Kirchner H. The production of a monocyte chemotactic factor by agammaglobulinemic chicken spleen cells. J Immunol. 1972 Nov;109(5):1149–1151. [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaese R. M., Strober W., Brown R. S., Waldmann T. A. The Wiskott-Aldrich syndrome. A disorder with a possible defect in antigen processing or recognition. Lancet. 1968 May 18;1(7551):1056–1061. doi: 10.1016/s0140-6736(68)91411-6. [DOI] [PubMed] [Google Scholar]
- Blaese R. M., Strober W., Levy A. L., Waldmann T. A. Hypercatabolism of IgG, IgA, IgM, and albumin in the Wiskott-Aldrich syndrome. A unique disorder of serum protein metabolism. J Clin Invest. 1971 Nov;50(11):2331–2338. doi: 10.1172/JCI106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Chae H. P., Lowman J. T., Krivit W., Good R. A. Wiskott-Aldrich syndrome. An immunologic deficiency disease involving the afferent limb of immunity. Am J Med. 1968 Apr;44(4):499–513. doi: 10.1016/0002-9343(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Immunodeficiency disease and malignancy. Various immunologic deficiencies of man and the role of immune processes in the control of malignant disease. Ann Intern Med. 1972 Oct;77(4):605–628. [PubMed] [Google Scholar]
- Oppenheim J. J., Blaese R. M., Waldmann T. A. Defective lymphocyte transformation and delayed hypersensitivity in Wiskott-Aldrich syndrome. J Immunol. 1970 Apr;104(4):835–844. [PubMed] [Google Scholar]
- Preud'Homme J. L., Griscelli C., Seligmann M. Immunoglobulins on the surface of lymphocytes in fifty patients with primary immunodeficiency diseases. Clin Immunol Immunopathol. 1973 Jan;1(2):241–256. doi: 10.1016/0090-1229(73)90025-1. [DOI] [PubMed] [Google Scholar]
- Sherwood G., Blaese R. M. Phytohaemagglutinin-induced cytotoxic effector lymphocyte function in patients with the Wiskott-Aldrich syndrome (WAS). Clin Exp Immunol. 1973 Apr;13(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Frankel A., Blaese R. M. Defective mononuclear leukocyte chemotaxis: a previously unrecognized immune dysfunction. Studies in a patient with chronic mucocutaneous candidiasis. Ann Intern Med. 1973 Apr;78(4):509–513. doi: 10.7326/0003-4819-78-4-509. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Spitler L. E., Levin A. S., Stites D. P., Fudenberg H. H., Pirofsky B., August C. S., Stiehm E. R., Hitzig W. H., Gatti R. A. The Wiskott-Aldrich syndrome. Results of transfer factor therapy. J Clin Invest. 1972 Dec;51(12):3216–3224. doi: 10.1172/JCI107148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden P. L., Blaese R. M., Strober W., Block J. B., Waldmann T. A. Impaired lymphocyte transformation in intestinal lymphangiectasia: evidence for at least two functionally distinct lymphocyte populations in man. J Clin Invest. 1972 Jun;51(6):1319–1325. doi: 10.1172/JCI106928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A. Wiskott-Aldrich syndrome: clinical, immunologic, and pathologic observations. J Pediatr. 1967 Feb;70(2):221–232. doi: 10.1016/s0022-3476(67)80417-7. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]



