Abstract
Objective:
We report our experience with hand-assisted laparoscopic nephroureterectomy (HALN) for upper urinary tract transitional cell carcinoma and compare our results with a contemporary series of open nephroureterectomy (ON) performed at our institution.
Methods:
Between August 1996 and May 2003, 90 patients underwent nephroureterectomy for upper-tract transitional cell carcinoma (TCC). Thirty-eight patients underwent HALN, while 52 had an ON. End-points of comparison included operative time, estimated blood loss (EBL), intraoperative and postoperative complications, length of hospital stay, pathologic grade and stage of tumor, and tumor recurrence.
Results:
The mean patient age was 72.3 and 70.6 years in the ON and HALN groups, respectively. Mean operative duration was 243 minutes (ON) and 244 minutes (HALN), with an EBL of 478mL in the open group versus 191mL in the hand-assisted group (P<0.001). No intraoperative complications occurred, but postoperative complications occurred in 4% and 11% of the ON and HALN groups, respectively (P=0.21). The mean hospital duration was 7.1 days (ON) versus 4.6 days (HALN) (P<0.01). No difference existed in the pathologic grade or stage distribution of urothelial tumors between the 2 groups. The mean follow-up was 51.0 months in the ON group and 31.7 months in the HALN group. Recurrence of urothelial carcinoma occurred in 50% of patients who underwent ON and 40% treated by HALN (P=0.38) at a median interval of 9.1 and 7.7 months, respectively, after surgery.
Conclusion:
Hand-assisted laparoscopic nephroureterectomy is an effective modality for the treatment of upper urinary tract urothelial carcinoma. Patients benefited from less intraoperative blood loss and a shorter hospitalization with an equivalent intermediate-term oncologic outcome compared with that of the open approach.
Keywords: Transitional cell carcinoma (TCC), Nephroureterectomy, Hand-assisted laparoscopy, Urologic oncology
INTRODUCTION
Transitional cell carcinoma (TCC) of the upper urinary tract (UUT) represents approximately 5% of urothelial malignancies and less than 10% of all renal tumors.1 Although open radical nephroureterectomy with excision of an ipsilateral periureteral bladder cuff has been considered the standard management for upper tract TCC,2 the operation is associated with a lengthy hospitalization and convalescence. The morbidity associated with the open approach has led to the investigation of minimally invasive techniques that may serve as alternatives.
Antegrade and retrograde endoscopic management of upper-tract TCC have been reported for treatment of this disease.3,4 However, the indications for these approaches are highly select and should be reserved for patients with a solitary kidney, renal insufficiency, bilateral disease, or low-volume tumors. The introduction of the laparoscopic nephroureterectomy, first performed in 1991 at Washington University,5 has provided another minimally invasive alternative to open surgery. Compared with open nephroureterectomy, early results with the laparoscopic approach were encouraging with decreased postoperative analgesia requirements, shorter hospitalization, better cosmesis, and improved convalescence.6 Despite these patient advantages, drawbacks of the laparoscopic approach include the lengthy operative time, the steep learning curve, and the need for a skilled laparoscopic surgeon.
The hand-assisted transperitoneal laparoscopic approach has been part of the evolution in minimally invasive alternatives for patients with upper-tract TCC. Keeley and colleagues7 reported the initial results with this approach and noted a significantly shorter operative duration than their series of laparoscopic nephroureterectomy. Several other small series have subsequently demonstrated the safety and efficacy of this procedure with comparable short-term oncologic control.8,9
To date, 38 patients with upper-tract transitional cell carcinoma have been treated with a hand-assisted transperitoneal laparoscopic nephroureterectomy (HALN) at our institution. We report our single institution experience and compare our outcomes data with that of a contemporary series of 52 consecutive patients who underwent an open radical nephroureterectomy (ON).
METHODS
Patient Selection
We retrospectively reviewed the records of 90 consecutive patients who underwent an open (ON) or hand-assisted laparoscopic transperitoneal (HALN) radical nephroureterectomy between August 1996 and May 2003 at the New York-Presbyterian Hospital. Thirty-eight patients underwent HALN, while 52 had an ON. All laparoscopic procedures were performed by 1 of 2 surgeons (JDP and RES), while various surgeons were involved in the open procedures.
Diagnosis of upper-tract transitional cell carcinoma was established by computed tomography, excretory urogram, retrograde ureteropyelogram, and/or ureteroscopy with tissue biopsies. Postoperative follow-up comprised regular interval history and physical examination, urinary cytologies, chest x-rays, and abdominopelvic CT scan. Surveillance cystoscopy was performed every 3 months for 2 years and every 6 months for the next 2 years. Demographic, operative, and follow-up data were recorded and compared between the 2 groups.
Demographic information of these 90 patients is summarized in Table 1.
Table 1.
Demographic Data
| HALN* | ON* | P Value | |
|---|---|---|---|
| No. Patients | 38 | 52 | — |
| Age (range) | 71 (43–79) | 72 (54–82) | 0.87 |
| Sex (M/F) | 23/15 | 26/26 | 0.32 |
| Mean ASA Class | 2.8 | 2.6 | 0.07 |
| Presenting sx (%) | |||
| Hematuria | 31(82) | 37(71) | 0.31 |
| TCC* surveillance† | 13(25) | 37(71) | 0.29 |
| Systemic symptoms | 1(3) | 0(0) | 0.24 |
| Incidental | 0(0) | 2(4) | 0.22 |
| Location of Tumor | |||
| Right/Left | 22/16 | 29/23 | 0.84 |
| Renal Pelvis | 25(66) | 24(46) | 0.06 |
| Upper Ureter | 2(5) | 3(6) | 0.92 |
| Mid Ureter | 1(3) | 3(6) | 0.48 |
| Lower Ureter | 6(16) | 12(23) | 0.39 |
| Multifocal | 4(11) | 10(19) | 0.26 |
HALN=hand-assisted laparoscopic nephroureterectomy; ON= open nephroureterectomy; TCC=transitional cell carcinoma.
Patients with a history of bladder cancer with surveillance studies revealing an upper urinary tract lesion.
Operative Technique
Patients in each group underwent a bowel preparation with a clear liquid diet and a bottle of magnesium citrate. Perioperative antibiotics were infused in the operating room, and general anesthesia with endotracheal intubation was used in all cases. An oral gastric tube was used to decompress the stomach, and venous insufflation boots were used to prevent lower extremity stasis.
Hand-Assisted Laparoscopic Nephroureterectomy (HALN)
The patient was placed in the flank position with adequate padding for the brachial plexus and the dependent hip, knees, and ankles. The lower leg was flexed while the upper leg was gently extended and a pillow placed between them. The operating table was flexed and the kidney rest was raised.
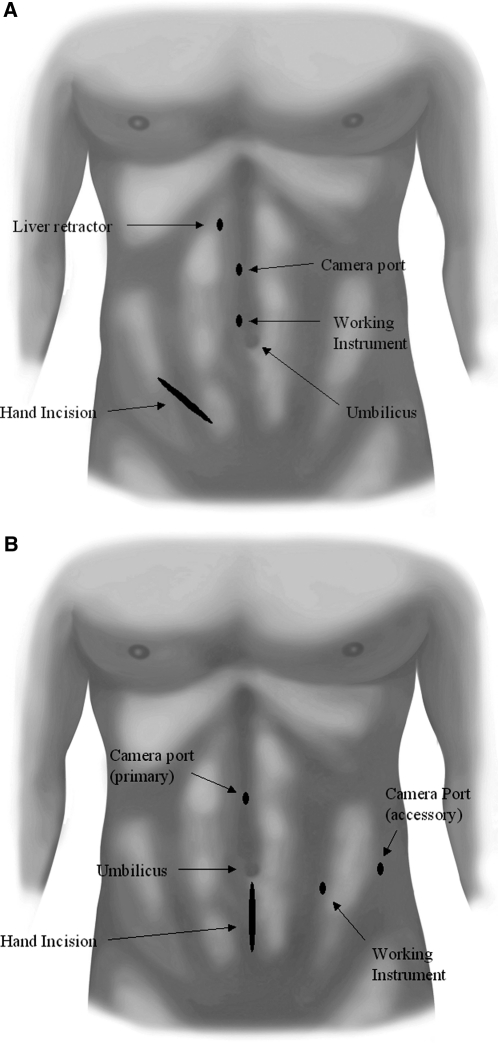
For right-sided HALN, 4 ports were utilized: a 12-mm trocar at the midclavicular line 2cm below the umbilicus (working instrument), a 5-mm periumbilical trocar (working instrument), a 5-mm trocar at the midline 5-cm above the umbilicus (camera), and a second 5-mm trocar just under the costal margin for the liver retractor. A 7-cm right lower quadrant incision was utilized for the hand-assist device. Left-sided HALN utilized a 12-mm trocar at the midclavicular line 2-cm inferior to the umbilicus (working instrument), a 5-mm trocar adjacent to the umbilicus (working instrument), a 5-mm trocar at the midline 2 finger breaths below the xyphoid process (camera), and a 7-cm vertical lower midline incision for the hand device (Figure 1).
Figure 1.
Trocar configuration and hand-assist device placement for (A) right and (B) left hand-assisted laparoscopic nephroureterectomy
For the right nephroureterectomy, the right colon and hepatic flexure were medially mobilized, and the right lobe of the liver was released from the body wall. The coronary ligament was incised to expose the upper pole of the kidney and the inferior vena cava. The duodenum was mobilized medially with the Kocher maneuver for exposure of the renal hilum. The renal hilum was then adequately dissected for identification of the renal artery and vein. The artery and then the renal vein were divided using the Endo-GIA vascular stapler. The remaining attachments of the kidney were then dissected from surrounding tissues with the Harmonic scalpel. Gerota's fascia was kept intact and the adrenal gland was left in place.
For left-sided nephroureterectomy, the colon was medialized from the level of the iliac vessels to the splenic flexure. The lateral splenic attachments were freed to the level of the gastric fundus. The colon, spleen, and pancreas were mobilized en bloc resulting in exposure of the anterior aspect of Gerota's fascia. In a similar fashion to the right, the hilum was exposed, the artery and then the vein were isolated and divided, and the kidney with Gerota's fascia intact was separated from the adrenal gland.
Management of the Distal Ureter and Bladder Cuff
The ureter was dissected to the level of the bladder by a combination of blunt dissection, clips, and electrocautery. The intramural ureter was managed by several different modalities as previously described.10 These techniques are briefly discussed below.
The open extravesical technique involves applying clips across the distal ureter to prevent tumor spillage during manipulation of the specimen. The hand-assist device is replaced by a self-retaining retractor, and traction is used to facilitate dissection of the transmural ureter. The detrusor muscle is then opened circumferentially from outside the bladder, and a 2-cm cuff of bladder with mucosa is removed en bloc with the distal ureter. The opening in the bladder is closed in 2 layers with 2– 0 and 3– 0 absorbable sutures.
The open intravesical technique was used in patients with evidence of distal ureteral malignancy. Surgical exposure of the bladder was obtained either through the hand-assist device incision, Pfannensteil, or Gibson incision. An anterior cystotomy was made and the ipsilateral ureteral orifice was circumferentially excised with traction placed on the extravesical ureter. Two centimeters of bladder cuff was removed, and these defects, as well as the anterior cystotomy, were closed in 2 layers with absorbable sutures.
In the cystoscopic technique, the ureter was completely mobilized to the level of the bladder laparoscopically. An endoscopic stapling device was used to transect the distal ureter across the detrusor muscle. The patient was then repositioned in the dorsal lithotomy position. The ipsilateral ureteral orifice was unroofed with a Collins knife attached to a resectoscope on cutting current. The incision was carried through the detrusor wall until extravesical fat, and the staple line were cauterized. A Foley catheter was left in place for a minimum of 7 days with the defect healing primarily.
Open Nephroureterectomy (ON)
The open surgical approach used an extraperitoneal flank approach to remove the kidney and the upper ureter. The distal ureter was excised either through a Pfannenstiel or a Gibson incision with similar management of the intramural ureter as described above.
Outcomes
End-points of comparison included operative duration, estimated blood loss (EBL), intraoperative and postoperative complications, length of hospital stay, pathologic grade and stage of tumor, and tumor recurrence. Statistical analysis was performed using the unpaired, 2-tailed Student t test and the chi-square (χ2) log-rank test with the Yates correction factor. The Excel 2000 (Microsoft, Redmond, Washington) and SAS for Windows, version 9.1 (SAS Institute, Cary, North Carolina) software programs were used for statistical calculations.
RESULTS
Table 1 shows the demographics of patients in the 2 operative groups. No significant difference existed between the hand-assisted laparoscopic and open surgical groups with respect to patient age, gender distribution, preoperative American Society of Anesthesiology (ASA) score, presenting symptoms, or tumor location. There was also no difference between the number of left-sided and right-sided surgical procedures performed between the groups.
Operative and Postoperative Outcomes
The hand-assisted laparoscopic group benefited from less blood loss (191 mL vs 478 mL) and a shorter hospital duration (4.6 vs 7.1 days) with an almost identical mean operative duration (244 vs 243 minutes) (Table 2). No conversions were needed from the hand-assisted approach to the open surgical approach. Of the hand-assisted group, no intraoperative complications occurred; however, 4/38 (11%) patients had a postoperative complication. Two patients had postoperative bleeding (one requiring re-exploration on postoperative day 1 without identification of a distinct source), one patient developed an enterocutaneous fistula that was managed conservatively by parenteral nutrition, and another patient had a myocardial infarction that required cardiac catheterization and angioplasty with coronary stenting. No mortalities occurred in this group. The open surgical group also had no intraoperative complications, but 2 patients had complications postoperatively. One patient developed an occipital cerebrovascular infarction requiring postoperative anticoagulation with no residual deficits at this time, and the other patient had a postoperative arrhythmia requiring pacemaker placement. There was no significant difference in the complication rate between the hand-assisted and open surgical groups (11% vs 4%, P=0.65).
Table 2.
Comparison of Hand-Assisted Laparoscopic Nephroureterectomy and Open Nephroureterectomy Operative and Postoperative Data
| HALN* | ON* | P Value | |
|---|---|---|---|
| Operative Duration (min) (range) | 244 (90-50) | 243 (50-400) | 0.91 |
| Estimated Blood Loss (mL) (range) | 191 (25–475) | 478 (100–2200) | <0.001 |
| Complications (%) | |||
| Intraoperative | 0 (0) | 0 (0) | |
| Postoperative | 4 (11) | 2 (4) | 0.65 |
| Management of Distal Ureter | |||
| Extravesical bladder cuff | 22 (58) | 32 (55) | 0.79 |
| Intravesical bladder cuff | 8 (21) | 20 (34) | 0.08 |
| TUR* unroofing of ureteral orifice | 8 (21) | 0 (0) | <0.001 |
| Hospital Days (range) | 4.6 (2–8) | 7.1 (4–13) | <0.01 |
HALN=hand-assisted laparoscopic nephroureterectomy; ON= open nephroureterectomy; TUR=transurethral resection.
Oncologic Outcomes
Pathologic evaluation confirmed that all tumors were transitional cell carcinoma. There was no difference in the pathologic grade and stage distribution of tumors between the 2 surgical groups (Table 3). The mean follow-up for the HALN group was significantly shorter (31.7 vs 51.0 months) than the ON group. This was expected as the HALN was first performed at our institution in 1999, and our series reflects the evolution of the laparoscopic experience since that time. At a mean follow-up of 31.7 months and 51.0 months, respectively, 58% (22/38) of the HALN and 44% (23/52) of the ON group had no evidence of disease recurrence.
Table 3.
Pathologic and Follow-up Data
| HALN* | ON* | P Value | |
|---|---|---|---|
| Mean Follow-up mos (range) | 31.7 (8–47) | 51.0 (13–135) | <0.05 |
| Pathologic Grade (%) | |||
| Low (I and II) | 23 (60) | 33 (63) | 0.78 |
| High (III) | 15 (40) | 19 (37) | |
| Pathologic Stage (%) | |||
| Superficial (Ta+Tis+T1) | 27 (71) | 41 (79) | 0.67 |
| Invasive (T2+T3+T4) | 9 (29) | 11 (21) | |
| Mean Interval to Recurrence (mos) | 7.7 | 9.1 | 0.55 |
| Recurrence of TCC* (%) | |||
| Bladder | 11 (29) | 18 (35) | 0.32 |
| Contralateral ureter | 1 (3) | 2 (4) | |
| Urethra | 1 (3) | 0 (0) | |
| Metastatic | 2 (6) | 6 (11) | |
| Current Disease Status* | |||
| NED | 22 (58) | 23 (44) | 0.78 |
| AWD | 14 (37) | 17 (33) | 0.69 |
| DOD | 1 (3) | 9 (17) | 0.03 |
| DWOD | 1 (3) | 3 (6) | 0.51 |
HALN=hand-assisted laparoscopic nephroureterectomy; ON= open nephroureterectomy; TCC=transitional cell carcinoma; NED=no evidence of disease; AWD=alive with disease; DOD=dead of disease; DWOD=dead without disease.
In the HALN group, the overall recurrence rate of TCC in our series was 40% (15 of 38 patients) at a mean interval of 7.7 months after surgery. Of the 15 recurrences, 11 occurred in the bladder. Eight of these 11 patients had a history of transitional cell carcinoma of the bladder that was present before the diagnosis of upper-tract disease. In considering the management of the distal ureter, no difference was noted in bladder recurrences based on whether the technique was extravesical,6 intravesical,2 or cystoscopic3 (P=0.65). Also no recurrences were noted at the port sites, incision sites, or in the location of the resected distal ureter. Metastasis to the liver developed in 2 patients. The ON group had a 50% overall recurrence rate (26 of 52 patients) at a mean interval of 9.1 months after surgery. Eighteen of these recurrences were in the bladder, and 10 of these patients had a prior history of bladder TCC. Once again, the management of the distal ureter had no bearing on the bladder tumor recurrence rate. Metastasis to the liver occurred in one patient.
DISCUSSION
Historically, the standard management of upper urinary tract transitional cell carcinoma has been open nephroureterectomy with excision of an ipsilateral bladder cuff. Usually, a single large incision or 2 incisions in the flank and abdomen are utilized. The morbidity, prolonged patient convalescence, and duration of hospitalization have been documented and are significant. The introduction of laparoscopic techniques was developed to reduce the morbidity of the procedure while maintaining the basic principles of surgical oncology.
Multiple series have documented that laparoscopic radical nephroureterectomy has helped improve patient convalescence and decrease hospitalization when compared with the open surgical approach.6,11 However,
the procedure is technically difficult and is associated with a steep learning curve. We feel that the hand-assisted approach offers several advantages over the pure laparoscopic approach. Firstly, it allows the less experienced urologist a more feasible alternative to nephroureterectomy than open surgery. Having a hand in the operative field allows for tactile sensation, spatial orientation, and blunt dissection, which are currently limited using the standard laparoscopic technique. It may be the most reasonable alternative to open surgery for urologists not facile with pure laparoscopic surgery. The other advantage is the ability to remove the specimen intact without requiring tissue morcellation. In a comparison of hand-assisted and standard laparoscopic nephroureterectomy, Landman and colleagues found that HALN decreased operative times without altering short-term measures of convalescence compared with standard laparoscopy.12
To date, several series have noted the effectiveness of transperitoneal hand-assisted laparoscopic surgery for managing upper-tract TCC. In a prospective, nonrandomized study, Seifman and colleagues noted a longer operative duration, but shorter hospitalization, with similar short-term oncologic control and hospital costs when comparing hand-assisted laparoscopic to open nephroureterectomy.13 Several other groups have provided similar data reflecting these variables.8,9,14,15 Our series is the largest to date with the longest follow-up that has compared the transperitoneal hand-assisted laparoscopic approach to the open approach for nephroureterectomy. We found that while there was a comparable operative duration between the 2 procedures, the hand-assisted group benefiting from less blood loss (191 mL vs 478 mL) and shorter hospital duration (4.6 vs 7.1 days). No differences existed in the intraoperative or postoperative complication rates between the groups, and no patients had positive surgical margins. From an oncologic perspective, almost 60% of patients treated by HALN had no evidence of disease at a mean follow-up of 2.5 years. The majority of TCC recurrences occurred in the bladder, thus reflecting the field change effect in the urothelium. Given the high intravesical recurrence rate in our series, it is imperative that these patients have close surveillance following management of the primary upper-tract TCC. When comparing the HALN and ON groups, no significant difference was found in either the TCC recurrence rate (40% vs 50%) or the interval to recurrence (7.7 vs 9.1 months). The higher recurrence rate and mortality rate in the ON group is most likely a function of the longer follow-up in this patient cohort. In fact, actuarial disease specific survivals show a similar 5-year disease specific survival (81% HALN vs. 77% ON, P=0.81) between the 2 surgical groups (data not shown).
The commonly reported techniques for treating the distal ureter and bladder cuff include extravesical excision with bladder cuff, intravesical excision with bladder cuff, and cystoscopic unroofing of the intramural tunnel with extravesical excision of the distal ureter.10 The hand-assisted approach affords a significant advantage in that the surgeon can facilitate dissection and resection by providing gentle countertraction on the distal ureter. Our series noted no differences in TCC recurrence rates based on the management of the distal ureter. Of note, it is difficult to specifically compare each technique for management of the distal ureter given the propensity for development of bladder tumors in 30% to 50% of patients with negative margins following nephroureterectomy. Furthermore, the small number of patients in each subgroup of distal ureteral management makes it difficult to draw significant conclusions between efficacy of one technique versus another. In our experience, the extravesical ureteral excision through the original hand-assist port was the most time efficient approach as it avoided repositioning the patient or making a new incision. For distal ureteral tumors, however, we still prefer the intravesical approach to optimally visualize that a negative bladder cuff margin is obtained.
Arguments against the transperitoneal approach include the potential for intraperitoneal seeding of cancer cells, manipulation of bowel yielding a prolonged ileus, as well as the potential for an intraperitoneal urinoma or seroma.16 In our series of patients, no recurrences occurred in the peritoneal cavity, and there was no delay in the return of bowel function compared with that in a series performed with a retroperitoneoscopic approach. We acknowledge several limitations in this manuscript including the heterogeneity of surgeons performing the ON procedure, the limited cohort size in the HALN group, and the difference in follow-up interval between the 2 surgical groups. Clearly, as the HALN approach becomes increasingly performed, larger cohorts and longer-term follow-up will be available for comparison.
CONCLUSION
Hand-assisted laparoscopic transperitoneal radical nephroureterectomy is an effective surgical option for the management of upper urinary tract transitional cell carcinoma. Compared with open nephroureterectomy, patients benefited from less intraoperative blood loss and a shorter hospitalization with similar operative duration and intermediate-term cancer control. HALN successfully duplicates the open approach.
References:
- 1. Fraley EE. Cancer of the renal pelvis. In: Genitourinary Cancer. Skinner DG, deKernion JB. eds. Philadelphia: WB Saunders Co; 1978:134 [Google Scholar]
- 2. Cummings KB. Nephroureterectomy: rationale in the management of transitional cell carcinoma of the upper urinary tract. Urol Clin North Am. 1980; 7: 569–578 [PubMed] [Google Scholar]
- 3. Grossman HB, Schwartz SL, Konnak JW. Ureteroscopic treatment of urothelial carcinoma of the ureter and renal pelvis. J Urol. 1992; 148: 275–277 [DOI] [PubMed] [Google Scholar]
- 4. Smith AD, Orihuela E, Crowley AR. Percutaneous management of renal pelvic tumors: a treatment option in selected cases. J Urol. 1987; 137: 852–856 [DOI] [PubMed] [Google Scholar]
- 5. Clayman RV, Kavoussi LR, Figenshau RS, et al. Laparoscopic nephroureterectomy: initial case report. J Laparoendoscopic Surg. 1991; 1: 343–349 [DOI] [PubMed] [Google Scholar]
- 6. Shalhav AL, Dunn MD, Portis AJ, et al. Laparoscopic nephroureterectomy for upper tract transitional cell cancer: the Washington University experience [review]. J Urol. 2000; 163: 1100–1104 [PubMed] [Google Scholar]
- 7. Keeley FX, Sharma NK, Tolley DA. Hand-assisted laparoscopic nephroureterectomy. BJU Int. 1999; 83: 504–505 [DOI] [PubMed] [Google Scholar]
- 8. Stifelman MD, Sosa RE, Andrade A, et al. Hand-assisted laparoscopic nephroureterectomy for the treatment of transitional cell carcinoma of the upper urinary tract. Urology. 2000; 56: 741–747 [DOI] [PubMed] [Google Scholar]
- 9. Jarrett TW, Chan DY, Cadeddu JA, et al. Laparoscopic nephroureterectomy for the treatment of transitional cell carcinoma of the upper urinary tract. Urology. 2001; 57: 448–453 [DOI] [PubMed] [Google Scholar]
- 10. Munver R, Del Pizzo JJ, Sosa RE. Hand-assisted laparoscopic nephroureterectomy for upper urinary-tract transitional-cell carcinoma [review]. J Endourol. 2004; 18: 351–358 [DOI] [PubMed] [Google Scholar]
- 11. McNeill SA, Chrisofos M, Tolley DA. The long-term outcome after laparoscopic nephroureterectomy: a comparison with open nephroureterectomy. BJU Int. 2000; 86: 619–623 [DOI] [PubMed] [Google Scholar]
- 12. Landman J, Lev RY, Bhayani S, et al. Comparison of hand assisted and standard laparoscopic radical nephroureterectomy for the management of localized transitional cell carcinoma. J Urol. 2002; 167: 2387–2391 [PubMed] [Google Scholar]
- 13. Seifman BD, Montie JE, Wolf JS., Jr Prospective comparison between hand-assisted laparoscopic and open surgical nephroureterectomy for urothelial carcinoma. Urology. 2001; 57: 133–137 [DOI] [PubMed] [Google Scholar]
- 14. McGinnis DE, Trabulsi EJ, Gomella LG, et al. Hand-assisted laparoscopic nephroureterectomy: description of technique. Techn Urol. 2001; 7: 7–11 [PubMed] [Google Scholar]
- 15. Li CC, Chou YH, Shen JT, et al. Comparison of hand-assisted laparoscopic nephroureterectomy with open surgery for upper urinary tract tumor. Kaohsiung J Med Sci. 2001; 17: 615–619 [PubMed] [Google Scholar]
- 16. Gill IS, Sung GT, Hobart MG, et al. Laparoscopic radical nephroureterectomy for upper tract transitional cell carcinoma: the Cleveland Clinic experience. J Urol. 2000; 164: 1513–1522 [PubMed] [Google Scholar]