Abstract
Objective:
This study analyses the technique and complications from total laparoscopic hysterectomy.
Methods:
Retrospective chart abstraction was performed on 830 consecutive patients operated on between 1996 and 2006. Demographic and surgical data were analyzed by ANOVA, chi-square, and Spearman and Pearson correlation techniques were used with significance set at P<0.05.
Results:
Of 830 consecutive patients, 5 (0.6%) were converted to laparotomy. Patients had a mean age of 50 (±11) years, a mean of 1.3 (±1.3) pregnancies, and a mean BMI of 27.6 (±6.8) kg/m2. The mean surgical duration was 132 (±55) minutes, with mean blood loss of 130 (±189) mL and average hospital stay of 1.4 (±0.9) days. Duration of surgery, blood loss, and hospital stay all decreased with the surgeon's increasing experience. Reoperative complications occurred in 38 patients (4.7%). Urologic injuries were observed in 23 patients (2.6%), with 9 (1.1%) requiring reoperation.
Conclusions:
This technique for TLH offers the benefits of minimally invasive surgery for patients needing hysterectomy, even those without vaginal capacity and uterine prolapse.
Keywords: Laparoscopy, Hysterectomy, Complications
INTRODUCTION
Type VII total laparoscopic hysterectomy (TLH) is a laparoscopic hysterectomy in which all of the surgical dissections, ligations, and sutures are completed entirely through the trocars, including the closure of the vagina.1 When compared with abdominal hysterectomy (AH) and laparoscopic-assisted vaginal hysterectomies (LAVH), TLH has been reported to result in shorter procedure durations,2 lower blood losses,3 and shorter hospital stays.3 Typically, vaginal hysterectomy (VH) and LAVH are performed in patients with at least moderate prolapse usually associated with parity, but some of these patients may later develop vaginal prolapse or incontinence.4 One recent report5 describes recurrence of prolapse in 12% of patients having a vaginal hysterectomy with vaginal vault suspension compared with 4% having a laparoscopic uterosacral ligament uterine suspension. Among nulliparous patients, the vaginal dissection may be difficult due to absence of prolapse and small vaginal capacity and may result in more complications.6
TLH may offer a minimal blood loss, short hospital stay, and be practicable in most women with minimal risk of complications, but randomized trials are lacking. A recent Cochrane meta-analysis of hysterectomy approaches reviewed 24 randomized trials of 3643 women, but only 2 trials with 71 women were identified for type VII TLH. “Very few contemporary newly trained gynecologists will have sufficient expertise and confidence to tackle TLH, which requires the highest level of surgical skill.”7 They concluded that “[t]he newest approach to hysterectomy (TLH) should be further evaluated.”7
It is speculated that if a TLH technique used familiar open technique standards and was observed to be safe in a large retrospective series, potentially more surgeons would learn and safely perform the technique, and randomized controlled trials could be designed to compare the various routes. In this article, technique and complications are reviewed and analyzed in great detail so that surgeons learning and newly performing TLH may avoid complications, and subsequent randomized trials could be planned.
METHODS
All TLH cases were identified from computerized billing lists by using the Current Procedure Terminology Codes8 for all types of hysterectomy. Approval by the Investigation Review Board at Sequoia Hospital in Redwood City, California, has been maintained with yearly updates. All consecutive, eligible patients were offered simple TLH for every benign gynecologic indication, Stage Ia1 (<3×7 mm invasion) cervical cancer, occult ovarian cancer, and clinical Stage IIA or less endometrial cancer. Patients were ineligible if they had documented severe abdominal adhesions from a prior operative report, or endometrial cancer in a uterus too large for intact removal through the vagina. Every surgery was performed by the author (KAO'H) from September 5, 1996 to April 1, 2006, at 4 California hospitals, assisted by a categorical obstetrics and gynecology resident, a gynecologist, or a general surgeon.
For clarification in this article, the technique used for this type VII TLH is described. The patient is in modified lithotomy position with hips extended, in a 40-degree angle Trendelenburg position. Shoulder bolsters prevent slippage up the table, and the arms are padded and tucked by the side. After the examination with the patient anesthetized, a Humi uterine manipulator (Cooper Surgical, Inc. Trumbull, CT, USA) is inserted, unless the patient has endometrial cancer, in which case the manipulator is inserted after laparoscopic washings and bipolar cautery occlusion of the proximal fallopian tubes.
After visual inspection of the ureter at the pelvic brim, the infundibulopelvic ligament, or, utero-ovarian ligament is coagulated and incised with an Ultrasonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA). Immediately after the round ligament is incised, the uterus, on the uterine manipulator, is pushed cephalad to recreate the “traction-counter-traction” concept of open surgical dissection of the lower uterine segment. This elevates the uterine arteries along the lower cervix away from the ureters. A bladder flap is incised and the anterior cervical fascia is exposed with blunt dissection off of the cervix broadly below the cervicovaginal margin. The uterine arteries are coagulated with a bipolar cautery at the mid lower cervical length, and then incised with the Ultrasonic scalpel. The uterine arteries are pushed downward to expose the cardinal ligament fibers attaching the arteries to the cylindrical cervical fascia; then the cardinal fibers are incised posteriorly to the uterosacral ligaments, and inferiorly, identifying the cervicovaginal margin as the lowest limit of dissection. The cervicovaginal margin is laparoscopically “palpated” with the laparoscopic instruments by using visual and manual cues to delineate the posterior margin, the lateral edges, and the anterior margin of the cervical stroma (Figure 1). Typically, the cervical stroma is firm and moves as a solid object, and the vaginal wall is pliant and dimples with pressure. If the cervicovaginal margin is not obvious, a thin malleable ribbon is inserted into the anterior vagina and elevated, identifying the anterior-most cervicovaginal margin. Then the Ultrasonic scalpel incises into the vagina at the precise margin of the cervix and vagina (Figure 2). When the pneumoperitoneum is lost, the uterine manipulator is removed and a surgical glove containing 2 fluffed 4×4 gauze sponges is inserted to obturate the lower vagina. Two Semb toothed cupped graspers (#A5261, Olympus Surgical, Orangeburg, NY, USA) are used to expose the cervicovaginal margin for Ultrasonic incision around the cervix. A tenaculum is inserted through the vagina (under or beside the glove) to grasp the cervix and remove the uterus. When the uterus is large, but only in the absence of endometrial cancer, it is morcellated transvaginally. Any pelvic mass is placed intact in a Lapsac surgical tissue pouch (#054100, Cook Urological, Spencer, IN, USA) for frozen section. The edges of the pouch are brought out through the introitus, and a speculum is inserted into the pouch, allowing puncture or morcellation and removal of the mass with no intraperitoneal spillage. The vaginal apex is closed with #1 polygalactic acid JK10 (Ethicon Endo-Surgery, Cincinnati, OH, USA) suture in 3 or more figure-of-eight (technically spiral) sutures with Semb graspers and a Wolfe needle driver (#8393.502, Richard Wolf Surgical Instruments, Vernon Hills, IL, USA), fixing the vaginal angle to the uterosacral ligaments for suspension.
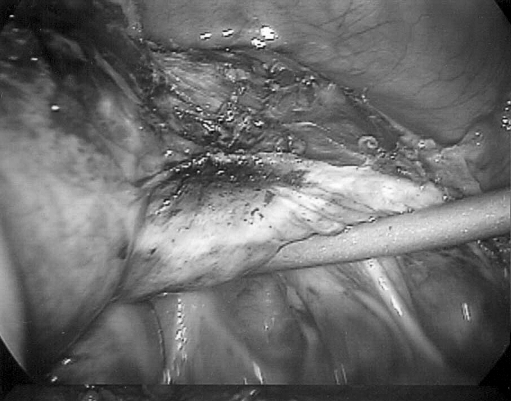
Figure 1.
The right lower uterine segment is seen with suction irrigator delineating the posterior cervicovaginal margin. The anterior cervical fascia is exposed well below the cervicovaginal margin. The uterine arteries have been coagulated with the bipolar and are ready for incision.
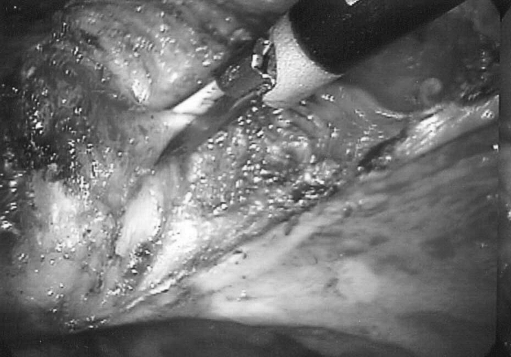
Figure 2.
After incising the cardinal ligament, the pliant vaginal wall is palpated laterally, anteriorly, and posteriorly to confirm the location of the cervicovaginal margin. Then, the vaginal wall is incised into with the Ultrasonic scalpel.
Descriptive statistics were generated related to sample characteristics and other variables of interest. ANOVA, chi-square, and Spearman and Pearson correlation techniques were used with significance set at P<0.05.
RESULTS
Of 830 patients eligible for this study, 5 were converted to open laparotomy. Three had massive myomata in the lower uterine segment obstructing laparoscopic ligation of the uterine arteries. The fourth patient was converted to laparotomy to explore a limited retroperitoneal bleeding site that was identified as a likely trocar injury. The fifth patient had persistent bleeding of the left infundibulopelvic vessels that had retracted into the retroperitoneum. Thus, the conversion rate for failed total laparoscopic hysterectomy was 0.6%. The frequencies of the primary preoperative diagnoses are shown in Table 1. Additional procedures performed in 71% of patients are listed in Table 2.
Table 1.
Preoperative Diagnoses
| Diagnoses (717) | Number |
|---|---|
| Leiomyomata uteri | 258 (31%) |
| Pelvic mass | 168 (20%) |
| Pelvic pain | 80 (10%) |
| Endometrial carcinoma | 87 (10%) |
| Familial breast/ovary cancer | 42 (5%) |
| Gender identity dysphoria | 38 (5%) |
| Prolapse | 26 (3%) |
| Adenomyosis | 28 (3%) |
| Atypical endometrial hyperplasia | 26 (3%) |
| Cervical dysplasia | 21 (2%) |
| Cervical carcinoma | 17 (2%) |
| Stress incontinence | 14 (2%) |
| Ovarian carcinoma | 12 (2%) |
| Uterine sarcoma | 3 (0.4%) |
| Vaginal carcinoma | 2 (0.3%) |
| Choriocarcinoma | 1 (0.1%) |
Table 2.
Additional Procedures Performed With Total Laparoscopic Hysterectomy*
| Procedure | Number |
|---|---|
| Cystoscopy | 410 (48%) |
| None | 228 (27%) |
| Appendectomy | 257 (31%) |
| Lymph node dissection | 57 (7%) |
| Uterosacral ligament plication | 102 (12%) |
| Excision of endometriosis | 55 (7%) |
| Ureterolysis | 51 (6%) |
| Burch colpopexy/Transobsturator tape | 47 (6%) |
| Omentectomy | 23 (8%) |
| Posterior repair | 12 (2%) |
| Sigmoidoscopy | 6 (1%) |
| Cholecystectomy | 6 (1%) |
| Inguinal herniorrhaphy | 6 (1%) |
| Umbilical herniorrhaphy | 4 (1%) |
| Sacropexy | 3 (0.4%) |
| Ureteral stent placement | 6 (1%) |
Some patients had more than one procedure.
The preoperative diagnosis correlated with the postoperative diagnosis in all cases. There was no surprise diagnosis of malignancy in this series. All cases of malignancy were managed with strict standards of oncological principles: pelvic masses were removed intact in a ripstop nylon bag.9 Washings were always taken first. Uteri were removed without morcellation, or placed in a bag.10 Nodes were dissected whenever indicated.11 The surgical procedures performed were never compromised by the minimally invasive techniques.12
The typical patient in this series had a mean age of 50.1 years (SD=11.0; range, 21 to 90). The average parity was 1.3 (SD=1.3; range, 0 to 9) with 38.2% (n=315) of these women being nulliparous. Their mean body mass index (BMI) was 27.8 kg/m2 (SD=7; range, 16 to 71) with 57% (n=468) of the women being in the overweight or higher BMI range (>24.9kg/m2).
Surgical duration was abstracted from operating room records and included all gynecologic procedures and incidental appendectomy.13 Patients requiring staging lymphadenectomy and omentectomy for malignancy, or other general surgical procedure such as cholecystectomy were not included in the duration analysis. The mean surgery duration was 130 minutes (SD=56; range, 28 to 355). Over one fifth (23.7%) of patients' surgeries were completed in 90 minutes or less. Neither age (r=0.025, P=0.513), body mass index (r=0.060, P=0.115), nor parity (t=1.896, P=0.058) impacted duration, but the increasing number of cases performed by the surgeon reduced duration (r=−0.448, P<0.001).
Blood loss was estimated by examining the contents in the canister before irrigation, or by surgeon and anesthesiologist agreement. The average blood loss was 130 mL (SD=178; range, 0 to 1200, median 75) with 16% of patients (n=132) losing less than an estimated 10mL of blood. This estimate is made when there is virtually no observable blood lost during the procedure, little or none observed in the pelvic cul de sac at the end of the procedure, and little or none lost from the laparoscopic incisions. More than half of the patients lost less than 50 mL of blood. While age (r =−0.018, P=0.633) and BMI (r=0.050, P=0.184) did not correlate with blood loss, lower parity (t =−2.91, P=0.004) and increasing experience (r=−0.247, P<0.001) correlated with reduced blood loss.
Transfusions, mostly related to complications, were needed in 3.0% of women (11.0 vs. 1.7%, P=0.0001), especially a reoperative complication (13 vs. 2.4%, P=0.0353). Age was not significantly related to having a transfusion (t=−0.09, P=0.93), nor was BMI (t=−0.7109, P=0.48). However, those requiring transfusions had longer surgery (t=−3.50, P=0.000) and more days in the hospital (t=−2.35, P=0.028).
The median uterine weight was 160, and the mean was 239 g, (SD = 291; range, 50 to 3131). Of the 632 with recorded uterine weights, 16% were between 250 grams and 499 g, and 12% were between 500 grams and 3131 g. BMI did not impact uterine weight (r=0.056, P=0.156); however, increasing age correlated with smaller uteri (r =−0.113, P=0.004). Having a larger uterus correlated with increased surgical time (r=0.268, P<0.001) and more blood loss (r=0.347, P<0.001). Seven nulliparous, obese patients with BMI ranging from 31 to 46, and massive uteri, had a TLH followed by a planned 6-cm Pfannenstiel incision used only for evacuation of the disconnected uterine tissue, weighing 1047 g to 3131 g. Only evacuation of tissue was performed through the Pfannenstiel incisions. The Pfannenstiel incisions were immediately closed, and the remainder of the case, including suture of the vaginotomy, was completed laparoscopically. Had any portion of these cases required laparotomy for completion of the hysterectomy/oophorectomy dissection, these cases would have been classified as “converted” or as a laparoscopic-assisted abdominal hysterectomy.1
The average hospital stay was 1.37 days (SD=0.97; range, 0 to 13, median, 1 day). Duration of hospital1 stay was recorded only by days, not hours; however, 73% of patients left the hospital by lunchtime the next day. Longer stays were not associated with age, BMI, parity, or uterine size, but did correlate with longer surgical times (r=0.347, P<0.001) and more blood loss (r=0.346, P<0.001). The length of hospital stay decreased with the increasing number of cases performed by the surgeon (r=−0.452, P<0.001).
Complications occurred in 83 patients (10%), and of those complications about half required reoperation (39 patients, 4.7%; Table 3). Urologic complications occurred in 23 (2.8%) patients and resulted in reoperation in about half (1.4%). Complications were not significantly associated with age, uterine weight, or parity. Complications were more likely to occur in women with a lower BMI (OR 0.95; 95% CI=0.90 to 0.99), with a high surgical blood loss (OR 1.002; 95% CI=1.001 to 1.003) resulting in longer operating times (OR 1.007; 95% CI=1.003 to 1.012) and a longer length of stay in the hospital (OR 1.78; 95% CI=1.39 to 2.28).
Table 3.
Complications
| Complication | Total N = 71 (9.9%) | No Reoperation N = 34 (4.7%) | Reoperation N = 37 (5.2%) |
|---|---|---|---|
| Urologic Injuries | |||
| Cystotomy, with repair | 12 (1.4%) | 10 (1.2%) | 2 (0.2%) |
| Bladder fistula, catheterized | 1 (0.1%) | 1 (0.1%) | |
| Ureterotomy, with Repair | 3 (0.4%) | 3 (0.4%) | |
| Ureter fistula, reimplanted | 4 (0.5%) | 4 (0.5%) | |
| Ureter fistula, stented | 3 (0.4%) | 3 (0.4%) | |
| Intestinal | |||
| Bowel injury from dissections | 3 (0.4%) | 1 (0.1%) | 2 (0.2%) |
| Adhesive bowel obstruction | 3 (0.4%) | 3 (0.4%) | |
| Hemorrhagic | |||
| Postoperative pelvic bleed | 5 (0.7%) | 5 (0.7%) | |
| Retroperitoneal hematoma | 6 (0.8%) | 6 (0.8%) | |
| Vaginal cuff bleed | 11 (1.3%) | 4 (0.6%) | 7 (0.8%) |
| Postoperative wound bleed | 1 (0.1%) | 1 (0.1%) | |
| Infectious | |||
| Pelvic cellulitis | 9 (1.1%) | 9 (1.1%) | |
| Pelvic seroma | 2 (0.3%) | 2 (0.3%) | |
| Pelvic abscess | 5 (0.4%) | 2 (0.1%) | 3 (0.3%) |
| Diverticulitis | 1 (0.1%) | 1 (0.1%) | |
| Wound Healing | |||
| 5-mm trocar hernia | 4 (0.6%) | 4 (0.6%) | |
| Vaginal dehiscence or injury | 5 (0.7%) | 2 (0.3%) | 3 (0.4%) |
| Pelvic suture granuloma | 1 (0.1%) | 1 (0.1%) | |
| Retained Surgical Device | 1 (0.1%) | 1 (0.1%) |
DISCUSSION
The demographics, diagnoses, and surgicopathological data reflect a broad diversity of consecutive patients and demonstrate a broad utility for TLH. Senior age and high BMI are not contraindications to TLH.12,14 Because type VII TLH does not depend on vaginal descensus, capacity, or laxity, minimally invasive hysterectomy is available to more women, including the nulliparous and obese. With a 38% nulliparity rate, few of these patients would have qualified for a VH or an LAVH. Agostini et al6 report a high rate of successful vaginal hysterectomy in their 52 nulliparous patients, but their complication rate was 13.46%, higher than our total complication rate for this subset (10.82%), and much higher than our reoperative rate (4.5%) in this subset. Their hemorrhage rate was 7.69%, which was much higher than our transfusion rate of 3.2%. This report responds to their conclusion that laparoscopic assistance for nulliparous women having hysterectomy “needed further investigation.” Their findings underscore the benefit of performing the entire surgery laparoscopically rather than struggling through a narrow vagina to reach a cervix with no descensus.
It is unfortunate that operating time for TLH only was not recorded in the operating room records. The mean operating time of 132 minutes is thus an overestimate of the time needed for TLH only. The duration decreased with surgeon's experience, with the last 100 cases taking 99 minutes or less. In controlled trials, type VII TLH has been observed to take the same or slightly more time than AH, similar or shorter time than LAVH, and 53 minutes longer than VH.7
Estimations of blood loss were estimations, per se. It is a weakness of this study that blood loss was not measured in a more precise fashion. In randomized trials, type VII TLH confers less blood loss than AH and LAVH do, and confers a similar blood loss as that of VH.7
Precise assessment of patient hospital stays would have required calculation of the hours from the end of surgery to the hour of discharge. Unfortunately, this was not done. In the first half of the series, patients stayed in the hospital 2 days; however, in the second half of the series, it became routine to discharge patients on postoperative day one. This has caused no problems, and no readmissions have been observed for an extra day of observation. Randomized trials reveal that type VII TLH confers a shorter hospital stay than do AH and LAVH, and a similar stay as that of VH.3,7
Uterine size did not correlate with increasing likelihood of complication (P=0.6705), urologic complication (P=0.5782), or conversion to laparotomy, as others have seen.15 Lower uterine segment fibroids in 3 cases precluded access to the uterine arteries necessitating conversion to laparotomy for bleeding control. Preoperative evaluation of the parametrium of patients with lower uterine segment fibroids can identify patients with an increased risk of laparotomy. Laparoscopic myomectomy for optimal visualization of the cervical anatomy is used when lower uterine fibroids are large.
The total complication rate of 9.8% and major complication rate of 4.5% in this series are comparable to rates in other TLH series16–18 (Table 3). Hoffman et al16 had a total and major complication rate of 10% and 5.6%, while Heinberg et al17 had a major complication rate of 14.4%, and Chapron18 reported complications in 10%.
A complication rate of 13.4% has been observed for VH performed on nulliparous women,6 and 18% for VH after Cesarean delivery.19 In randomized, controlled trials, type VII TLH conferred fewer complications, fewer wound infections, similar urologic complication rates, and was more cost effective than was AH,7 and resulted in comparable complications as LAVH20 and VH.7,21
Six categories of complications are analyzed for possible prevention strategies: urologic injuries, intestinal injuries, hemorrhagic events, infections, wound healing problems, and retained device complications.
Urologic Injuries
Nineteen (2.3%) patients had a urological injury, with half requiring reoperation: 3 cystoscopic ureteral stent placements, 4 ureteral reimplantations, and 2 laparotomies for closure of bladder fistula. TLH urological injury rates are reported at 2.5% to 3.4%12,17,22,23 and 2.2% in the first half of one series, decreasing to 0.9% in the second half.24
Ten patients had cystotomy with intraoperative suture repair. Trocar insertion piercing both walls of the bladder occurred in one patient. Six injuries occurred during bladder flap dissections in patients with large lower uterine fibroids (uterine weights all >510 g) and 3 with prior Cesarean deliveries. One injury occurred during dissection of the anterior abdominal wall peritoneum to access the space of Retzius for a Burch procedure. To repair these, a running 3- 0 polygalactic acid suture in 2 layers was used to provide watertight approximation.25 Surgical closure was confirmed by cystoscopy and followed by 7 days of catheter rest. It was successful in all but 2 patients who required laparotomy for successful repair after developing delayed breakdown of the laparoscopic cystotomy repair.
When the margins of the bladder are not obvious before any dissection, inflating the bladder with a small amount of carbon dioxide can effectively delineate the edges. By clamping a hemostat on the Foley catheter, attaching the insufflator tubing to it, and slowly opening the clamp while laparoscopically watching the bladder inflate with a small amount of carbon dioxide, the margins can be seen. Reconnecting the catheter tubing to the drainage bag easily deflates the bladder.
After low transverse Cesarean delivery, patients typically have scarring on the upper portion of the anterior cervix and lower uterine segment, extending as low as the middle level of the cervix, but rarely ever lower. To dissect below this scar to unoperated vesicocervical fascia, the broad ligament adventitia is opened widely along the anterolateral aspect of the cervix until below the scar, where the smooth anterior white fascia of the cervix is easily separated from the bladder. Then cutting ultrasonic dissection of the scar can be undertaken on the cervix upward, incising to reflect the bladder down.
Ten ureteral injuries occurred. Ureterotomy or ureteral transsection with immediate and successful repair occurred in 2 patients. In one case very early in the series (case #31), parametrial dissection landmarks were distorted by large lower uterine segment fibroids. In the other, redundant peritoneum was being trimmed after the completed hysterectomy for a massive, predominantly retroperitoneal fibroid uterus, without reconfirmation of the ureteral location. In both cases, a stent was inserted cystoscopically, and laparoscopically placed in the proximal portion of the ureter, with ureteroureterostomy by using 4 - 0 Vicryl suture on the spatulated ends, and peritoneal closure without a drain. Constant attention to the ureteral location would have prevented both injuries.
Eight ureteral fistulae developed postoperatively, most in the first third of the series, due to 2 sites of potential injury.It is well known that lack of recognition of the ureter in the lateral parametrial canal when ligating the uterine artery can result in ureteral injury 4cm to 5cm from the ureterovesical junction.
An additional site of ureteral injury has come about solely through TLH due to the lack of precise recognition of the cervicovaginal margin and dissection too far down the vagina. Dissection below the cervicovaginal margin can injure the ureter 2 cm to 3 cm from the ureterovesical junction. In open cases, surgeons have easily avoided this injury by carefully palpating the cervicovaginal margin between thumb and forefinger. Surgeons keep the uterus on traction-counter-traction during laparotomy and feel the cervicovaginal margin repeatedly before incising into the vagina precisely at the cervicovaginal margin. The standard for laparoscopic surgeons must be the same: always know the exact site of the cervicovaginal margin by “palpating” the cervicovaginal margin with laparoscopic instruments to feel the precise change from the firmness of the cervical body to the pliancy of the vaginal wall, anteriorly, posteriorly, and laterally. When any of these landmarks are in question, a long right-angled Heaney retractor or 1-inch ribbon is inserted to the anterior vagina and elevated, and the vaginotomy is performed safely onto the retractor's edge at the anterior central cervicovaginal margin.
Cystoscopy after TLH should be performed in any case in which bladder or ureteral integrity is in question, to confirm closure of any repaired defect or to confirm patency of ureters after procedures, such as uterosacral ligament plications or Burch colposuspensions.22 A 5-mm laparoscope can be used after instilling saline in the bladder with the suction irrigator. No indigo carmine is required to see the ureteral jets. Using the irrigator and laparoscope gently as described has resulted in no injuries.
Intestinal Injuries
Three intestinal injuries occurred in the series; only one was recognized immediately and repaired. One patient had slippage of the trocar outside of the abdominal cavity, with reinsertion performed without direct observation, through the bowel wall, requiring a figure-of-eight imbrication of both sites with 3- 0 polygalactic acid suture. The other 2 were occult injuries, presenting on days 7 and 29, possibly from a lighted scope left in the abdomen touching the bowel, or inadvertent injury from Ultrasonic scalpel dissection.
Three patients developed obstructive small-bowel adhesions to the vaginal apex, as evidenced by prolonged ileus and radiological demonstration of small-bowel obstruction at the vaginal apex. They each required laparoscopic lysis of adhesions for complete resolution.
Hemorrhagic Events
Vaginal hemorrhage, retroperitoneal hemorrhage, and intraabdominal hemorrhage occurred in 11 (1.3%), 6 (.8%), and 5 (.7%) patients, respectively, as observed in other series.24 Of the 11 patients with vaginal hemorrhage, 5 occurred in the recovery room with immediate return to the operating room for additional suture placement vaginally. Using at least 3 figure-of-eight (technically spiral) sutures along the vaginal apex seems essential, even when there is good hemostasis of the cuff edge. Six patients developed brisk arteriolar cuff bleeding on days 7 through 10, and 4 required sutures in the office. None of these 4 patients were diagnosed with a coagulopathy; however, the suture material appeared dissolved in each. Two patients had bleeding remote from the hospital and were observed in an emergency room without suture.
Of the 11 patients with abdominal or retroperitoneal hemorrhage, 6 were managed expectantly because they maintained normal vital signs or presented long after discharge with hematoma, which resolved. Two had unexpectedly intense pain in the first 12 hours after surgery, which might have been an early warning symptom. Five developed hypotension in the hospital and required transfusion and laparoscopic evacuation of hematoma. No bleeding sites were identified in any of the reoperative cases, and none required laparotomy.
Infections
Nine patients (1%) were diagnosed with pelvic cellulitis and received oral antibiotics with resolution of symptoms. Five (0.4%) patients were suspected of having pelvic abscess: 2 had 3.0-cm fluid collections with CT aspirate of clear sterile fluid. Three had abscess: 2 were laparoscopically irrigated and drained, while one patient required laparotomy. Diverticulitis prolonged the hospital stay of one patient treated with intravenous antibiotics. This infection rate is comparable to that of other reports on TLH.16,26
Wound Healing Problems
Four patients developed small-bowel herniation into a 5-mm trocar site, with 3 diagnosed after discharge from the hospital. Each patient developed nausea and abdominal distension; and CT was confirmatory in all cases. In each case, laparoscopic release resulted in recovery of bowel function after 3 days. The fascial defect was closed laparoscopically with 2- 0 prolene. Herniation into a 5-mm trocar may occur with more degrees of instrument manipulation stretching the fascial incisions.27
Five patients have had partial vaginal dehiscence, most after sexual penetration after the 6-week postoperative vaginal examination. One patient developed complete dehiscence of the vaginal apex 18 days after her surgery, and required operative repair. None of the other 4 patients required surgical repair, but all patients were advised to use a 1-cm thick foam ring around their partner's penis during sexual activity to prevent their deepest penetration for the next 3 months. All have done well since and do not still use the ring. There have been no further incidents since warning all patients of the 1% chance of rupture with deep penetration after 6 weeks.
Retained Device
An 8-mm segment of metal element from the bipolar cautery was retrieved laparoscopically from 1 patient in postoperative week 4.
Most of the gynecological, hemorrhagic, infectious, and general surgical complications were addressed laparoscopically, vaginally, or locally. While all of the urological complications were treated by laparotomy except for cystoscopic stenting, this statistic will likely shift in time to laparoscopic repair.28
CONCLUSION
This technique for type VII TLH appears safe and allows excellent access to the entire abdomen as needed in cancer surgeries, for patients with pelvic mass, endometriosis, pain, or adhesions with minimal morbidity. A total laparoscopic capability makes the benefits of a minimally invasive approach available to more women, including obese and nulliparous women. With an understanding of the complications from this technique, it is hoped that complications can be avoided and that more surgeons will safely learn TLH. Experienced laparoscopic surgeons are urged to initiate needed randomized clinical trials of type VII TLH.
Contributor Information
Katherine A. O'Hanlan, Gynecologic Oncology Associates, Palo Alto, California, USA..
Suzanne L. Dibble, University of California, San Francisco, California, USA..
Anne-Caroline Garnier, Duke University School of Medicine, Durham, North Carolina, USA..
Mirjam Leuchtenberger Reuland, Ruprecht-Karls-Universität, Heidelberg, Germany..
References:
- 1. Nezhat C, Nezhat F, Admon D, Nezhat AA. Proposed classification of hysterectomies involving laparoscopy. J Am Assoc Gynecol Laparosc. 1995;2(4):427–429 [DOI] [PubMed] [Google Scholar]
- 2. Lee PI, Lee YT, Lee SH, Chang YK. Advantages of total laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 3(4, Supplement):S24–S25, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Hasson HM, Rotman C, Rana N, Asakura H. Experience with laparoscopic hysterectomy. J Am Assoc Gynecol Laparosc. 1993;1(1):1–11 [DOI] [PubMed] [Google Scholar]
- 4. Barrington JW, Edwards G. Posthysterectomy vault prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(4):241–245 [DOI] [PubMed] [Google Scholar]
- 5. Diwan A, Rardin CR, Strohsnitter WC, Weld A, Rosenblatt P, Kohli N. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):79–83 [DOI] [PubMed] [Google Scholar]
- 6. Agostini A, Bretelle F, Cravello L, Maisonneuve AS, Roger V, Blanc B. Vaginal hysterectomy in nulliparous women without prolapse: a prospective comparative study. BJOG. 2003;110(5):515–518 [PubMed] [Google Scholar]
- 7. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005(1):CD003677. [DOI] [PubMed] [Google Scholar]
- 8. American Medical Association CPT® 2006 Professional Edition. Chicago, IL: American Medical Association; 2006 [Google Scholar]
- 9. O'Hanlan KA, Lopez L, Garnier A-C, Huang G, Leuchtenberger M. Total laparoscopic hysterectomy for adnexal pathology and body mass index [abstract]. Gynecol Oncol. 2003;88:243. [DOI] [PubMed] [Google Scholar]
- 10. O'Hanlan KA, Huang GS, Garnier AC, et al. Total laparoscopic hysterectomy versus total abdominal hysterectomy: cohort review of patients with uterine neoplasia. JSLS. 2005;9(3):277–286 [PMC free article] [PubMed] [Google Scholar]
- 11. O'Hanlan KA, O'Holleran MS. Surgicopathological data on 90 patients having total laparoscopic hysterectomy with and without staging lymphadenectomy for uterine neoplasia. Gynecol Oncol. 2006;101(1 Suppl):S120–121 [Google Scholar]
- 12. O'Hanlan KA, Huang GS, Lopez L, Garnier AC. Total laparoscopic hysterectomy for oncological indications with outcomes stratified by age. Gynecol Oncol. 2004;95(1):196–203 [DOI] [PubMed] [Google Scholar]
- 13. O'Hanlan KA, Fisher DT, O'Holleran MS. Total laparoscopic hysterectomy: 702 cases with outcomes stratified by incidental appendectomy. JSLS. In press [PMC free article] [PubMed] [Google Scholar]
- 14. O'Hanlan KA, Huang GS, Lopez L, Garnier AC. Selective incorporation of total laparoscopic hysterectomy for adnexal pathology and body mass index. Gynecol Oncol. 2004;93(1):137–143 [DOI] [PubMed] [Google Scholar]
- 15. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9(2):125–130 [DOI] [PubMed] [Google Scholar]
- 16. Hoffman CP, Kennedy J, Borschel L, Burchette R, Kidd A. Laparoscopic hysterectomy: the Kaiser Permanente San Diego experience. J Minim Invasive Gynecol. 2005;12(1):16–24 [DOI] [PubMed] [Google Scholar]
- 17. Chapron C, Dubuisson JB, Ansquer Y, Fernandez B. [Total hysterectomy for benign pathologies. Laparoscopic surgery does not seem to increase the risk of complications]. J Gynecol Obstet Biol Reprod (Paris). 1998;27(1):55–61 [PubMed] [Google Scholar]
- 18. Heinberg EM, Crawford BL, 3rd, Weitzen SH, Bonilla DJ. Total laparoscopic hysterectomy in obese versus nonobese patients. Obstet Gynecol. 2004;103(4):674–680 [DOI] [PubMed] [Google Scholar]
- 19. Boukerrou M, Lambaudie E, Collinet P, Crepin G, Cosson M. A history of cesareans is a risk factor in vaginal hysterectomies. Acta Obstet Gynecol Scand. Dec 82(12):1135–1139, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Cook JR, O'Shea RT, Seman EI. Laparovaginal hysterectomy: a decade of evolution. Aust N Z J Obstet Gynaecol. 2004;44(2):111–116 [DOI] [PubMed] [Google Scholar]
- 21. Ribeiro SC, Ribeiro RM, Santos NC, Pinotti JA. A randomized study of total abdominal, vaginal and laparoscopic hysterectomy. Int J Gynaecol Obstet. 2003;83(1):37–43 [DOI] [PubMed] [Google Scholar]
- 22. Ribeiro S, Reich H, Rosenberg J, Guglielminetti E, Vidali A. The value of intra-operative cystoscopy at the time of laparoscopic hysterectomy. Hum Reprod. 1999;14(7):1727–1729 [DOI] [PubMed] [Google Scholar]
- 23. Jaenisch JB, Junior WA. 100 total laparoscopic hysterectomies in private practice in Brazil [see comments]. J Am Assoc Gynecol Laparosc. 1999;6(2):169–171 [DOI] [PubMed] [Google Scholar]
- 24. Wattiez A, Soriano D, Cohen SB, et al. The learning curve of total laparoscopic hysterectomy: comparative analysis of 1647 cases. J Am Assoc Gynecol Laparosc. 2002;9(3):339–345 [DOI] [PubMed] [Google Scholar]
- 25. Lee CL, Lai YM, Soong YK. Management of urinary bladder injuries in laparoscopic assisted vaginal hysterectomy. Acta Obstet Gynecol Scand. 1996;75(2):174–177 [DOI] [PubMed] [Google Scholar]
- 26. Obermair A, Manolitsas TP, Leung Y, Hammond IG, McCartney AJ. Total laparoscopic hysterectomy versus total abdominal hysterectomy for obese women with endometrial cancer. Int J Gynecol Cancer. 2005;15(2):319–324 [DOI] [PubMed] [Google Scholar]
- 27. Nezhat FR, Nezhat CH, Seidman DS. Incisional hernias after advanced laparoscopic surgery. J Am Assoc Gynecol Laparosc. 3(4, Supplement):S34–35, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Giberti C, Germinale F, Lillo M, Bottino P, Simonato A, Carmignani G. Obstetric and gynaecological ureteric injuries: treatment and results. Br J Urol. 1996;77(1):21–26 [DOI] [PubMed] [Google Scholar]