Abstract
Background and Objectives:
Fossa navicularis strictures following radical prostatectomy are reported infrequently. We recently experienced a series of fossa strictures following robot-assisted laparoscopic radical prostatectomy. Fossa strictures are usually procedure-induced, arising from urethral trauma or infection; catheter size has not been reported as a factor. We describe herein our experience to determine and prevent fossa navicularis stricture development.
Methods:
From June 2002 until February 2005, 248 patients underwent robot-assisted laparoscopic prostatectomy with the da Vinci surgical system at our institution. Fossa strictures were diagnosed based on acute onset of obstructive voiding symptoms, IPSS and flow pattern changes, and bougie calibration. During our series, we switched from an 18F to a 22F catheter to avoid inadvertent stapling of the urethra when dividing the dorsal venous complex. All patients had an 18F catheter placed after the anastomosis for 1 week. Parameters were evaluated using Fisher's exact test and the Student t test for means.
Results:
The 18F catheter group (n=117) developed 1 fossa stricture, whereas the 22F catheter group (n=131) developed 9 fossa strictures (P=0.02). The fossa stricture rate in the 18F group was 0.9% versus 6.9% in the 22F group. The 2 groups had no differences in age, body mass index, cardiovascular disease, International Prostate Symptom Score, urinary bother score, SHIM score, preoperative PSA, operative time, estimated blood loss, cautery use, prostate size, or catheterization time.
Conclusions:
Using a larger urethral catheter size during intraoperative dissection appears to increase the risk 8-fold for fossa stricture as compared with the 18F catheter. The pneumoperitoneum and prolonged extreme Trendelenberg position could potentially contribute to local urethral ischemia.
Keywords: Laparoscopy, Urethral stricture, Robotic-assisted surgery, Robotics
INTRODUCTION
Fossa navicularis strictures following radical prostatectomy are rare. Unlike other anterior urethral strictures, fossa strictures are usually inflammatory or procedure-induced, arising from urethral trauma caused by endoscopic procedures, catheterizations, or subsequent infections.1 In the literature, catheter size has not been described as a factor. We describe its incidence in our robotic-assisted laparoscopic radical prostatectomy (RLP) series to determine its likely causal factors.
METHODS
The data of 248 men undergoing RLP from June 2002 until February 2005 were prospectively entered into an electronic database. Before surgery, all men were evaluated, and the following data entered: age, height, weight, International Prostate Symptom Score (IPSS), urinary bother score, Sexual Health Inventory in Men (SHIM) score, prostate-specific antigen (PSA), and pertinent medical history. Standard perioperative and postoperative parameters were evaluated. Urinary and functional outcomes were attained by self-administered questionnaires, including the 7-item IPSS, the 5-item SHIM, and selected questions from the 26-item Expanded Prostate Cancer Instrument Composite, at the routine 3-month and 9-month follow-up visits. The questionnaire asked whether patients wore pads, how many weeks it took to not need pads, how many weeks it took to return to work, and how many weeks it took to return to baseline energy levels. A non-clinical research associate (DWS) collected the follow-up information. Complications were defined by the need for prolongation of hospitalization, the need for a secondary procedure, or rehospitalization within 30 days. All statistical comparisons between the stricture and nonstricture groups as well as the 18F and 22F catheter groups were 2-sided using Fisher's exact test and the Student t test for means (Statistical Analysis Systems, version 8.2, statistical package). Multivariate analysis was also performed with a stepwise logistic regression; using the preoperative continuous variables, BMI, PSA, SHIM, EBL, IPSS, bother score, age, and prostate weight (Table 1) as independent variables in the prediction of a positive stricture. Ongoing institutional review board approval has been in place since 1998.
Table 1.
Demographic, Clinical, and Perioperative Data for Men Reporting Strictures Versus Stricture-Free Men
| Variable* | No Stricture | SE | Stricture | SE | P† |
|---|---|---|---|---|---|
| Patients (n) | 238 | 10 | |||
| Age (yr) | 62.0 (43–79) | 0.5 | 63.8 (51–71) | 2.4 | 0.45 |
| BMI | 27.0 (20.6–34.9) | 0.2 | 26.3 (24.2–34.0) | 0.7 | 0.52 |
| AUA symptom score | 8.5 (0–32) | 0.5 | 7.4 (1–27) | 1.8 | 0.59 |
| Urinary bother score | 1.8 (0–6) | 0.1 | 1.6 (0–6) | 0.5 | 0.81 |
| SHIM score | 17.8 (1–25) | 0.5 | 18.4 (1–25) | 2.6 | 0.82 |
| Preoperative PSA (ng/mL) | 7.1 (0.1–62.0)0.4 | 6.2 (1.8–18.0) | 1.5 | 0.65 | |
| Prostate size (g) | 50.1 (12.5–163.0) | 1.4 | 46.1 (20.0–69.1) | 3.3 | 0.29 |
| EBL (mL) | 106 (25–350) | 4.4 | 67.5 (25–400) | 16.3 | 0.07 |
BMI = body mass index; AUA = American Urological Association; SHIM = sexual health inventory in men; PSA = prostate-specific antigen; EBL = estimated blood loss; SE = standard error
Two-sided Fisher's exact test
All RLPs were performed in the same manner with the da Vinci surgical system (Intuitive Surgical Corp., Mountain-view, CA). Patients are placed supine in an extreme Trendelenburg position and undergo abdominal insufflation with a pneumoperitoneum of 15 mm Hg. It has been standard protocol to insert a silastic urethral catheter at the beginning of the procedure that is utilized throughout the dissection until completing the anastomosis. At this point, a new 18F silastic catheter is placed and is generally removed 7 days postoperatively. At case number 35, however, we switched from an 18F to a 22F silastic urethral catheter during the dissection as an aid to prevent stapling the urethra. We use a stapling device instead of suturing the dorsal venous complex based on findings from a previous study.2 In that study, the data demonstrated that the stapler technique provided a more defined apical dissection and a statistically significant reduction in positive margins in patients with organ-confined disease. We switched back to an 18F catheter at case number 166 due to concerns regarding strictures in the fossa navicularis.
Fossa strictures were diagnosed based on acute onset of obstructive lower urinary symptoms, IPSS and flow pattern changes, and bougie a boule calibration. Obstructive voiding symptoms included a decreased force of stream, dribbling or splaying, and prolonged voiding.
RESULTS
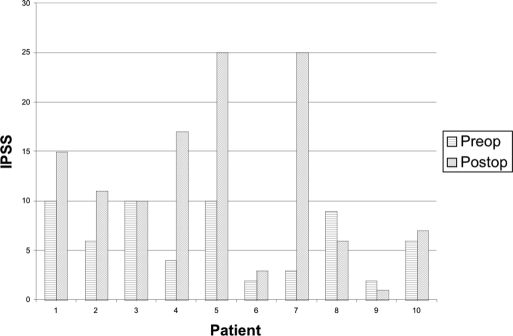
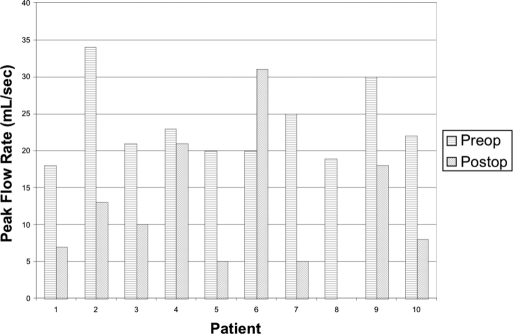
Figure 1 presents the preoperative and postoperative IPSS in men diagnosed with fossa navicularis strictures. IPSS increased in 8 of 10 men with a mean change of 7.7 (range, 1 to 22). Figure 2 depicts the preoperative and postoperative urinary peak flow rates in fossa stricture patients. Peak flow rates also decreased in 8 of 10 men with a mean change of 13.3 mL per second (range, 2 to 21). Postvoid residuals of stricture patients were minimal (range, 0 mL to 55 mL) with the exception of a patient who had acute urinary retention (420 mL). Flexible cystourethroscopy was performed on most stricture patients; however, retrograde urethrography was only performed on 2 men undergoing surgical repair.
Figure 1.
Preoperative and postoperative International Prostate Symptom Score (IPSS) in fossa stricture patients.
Figure 2.
Preoperative and postoperative peak flow rates in fossa stricture patients.
Table 1 presents the demographic, clinical, and perioperative data for men reporting strictures versus stricture-free men. The mean follow-up was 14.4 months for stricture patients versus 10.1 months for stricture-free patients, and 10.8 months (range, 3.6 to 33.6) for all patients. The groups were comparable for the standard clinical factors, such as age, body mass index (BMI), IPSS, urinary bother score, SHIM score, and preoperative PSA level. Also no difference existed between groups regarding operative time, prostate size, estimated blood loss (EBL), and cardiovascular disease. In addition, none in the stricture group had any history of a previous endoscopic procedure. Multivariate analysis also demonstrated that none of the preoperative continuous variables predicted the presence of strictures (all P>0.18).
Table 2 outlines the demographic, clinical, and perioperative data for men stratified by catheter size. The 18F and 22F catheter groups were also comparable for age, BMI, baseline IPSS, urinary bother score, SHIM score, preoperative PSA, operative time, prostate size, EBL, and complications. The 22F catheter group, however, had a significantly higher rate of stricture formation. When a 22F catheter was used, 6.9% of patients developed a stricture versus 0.9% of patients with an 18F catheter during the intraoperative dissection (P=0.02). The urethral diameter of the fossa strictures ranged from 6F to 14F by bougie a boule calibration. The mean length of urethral catheterization was 7.1 days (range, 7 to 8) in the stricture group with the mean time to stricture development at 50 days (range, 34 to 93). One patient in the stricture group developed a bladder neck contracture and another presented with a lower urinary tract infection.
Table 2.
Demographic, Clinical Data, and Perioperative Data for 18F and 22F Catheter Groups
| Variable* | 18 F | SE | 22 F | SE | P |
|---|---|---|---|---|---|
| Patients (n) | 117 | 131 | |||
| Age (yr) | 62.0 (46–79) | 0.6 | 62.2 (43–78) | 0.7 | 0.80 |
| BMI | 27.2 (20.6–40.0) | 0.3 | 26.8 | 0.3 | 0.38 |
| AUA symptom score | 8.7 (0–35) | 0.6 | 8.2 (0–31) | 0.6 | 0.58 |
| Urinary bother score | 1.8 (0–6) | 0.1 | 1.7 (0–6) | 0.1 | 0.65 |
| SHIM score | 18.5 (1–25) | 0.7 | 17.3 (1–25) | 0.7 | 0.24 |
| Preoperative PSA (ng/mL) | 6.7 (1.1–31.0) | 0.4 | 7.3 (0.1–62.0) | 0.6 | 0.46 |
| Prostate size (g) | 49.4 (12.5–163.0) | 1.8 | 50.5 (15.0–135.0) | 1.9 | 0.68 |
| EBL (mL) | 110.7 (25–350) | 6.6 | 98.9 (25–400) | 5.5 | 0.16 |
| Fossa stricture | 1 | 9 | 0.02† |
BMI = body mass index; AUA = American Urological Association; SHIM = sexual health inventory in men; PSA = prostate-specific antigen; EBL = estimated blood loss; SE = standard error
Two-sided Fisher's exact test
In addition, urethral reconstruction was required in 2 of 9 patients (22%) who developed strictures with the larger 22F catheter. Only one subject developed a stricture while using an 18F catheter and required only soft dilation as treatment. In the present series, 76% of RLP patients attained pad-free continence at 3 months. All comparisons made by the Student t test were also examined with the nonparametric Wilcoxon rank sum test, with similar conclusions.
DISCUSSION
After a thorough review of the literature, we noted that no articles specifically focused on fossa strictures, and most information relating to these strictures is secondary to discussion of more common strictures of the bulbar urethra. It has been reported that, generally, strictures involving the fossa have 3 distinct causes: inflammation, procedure-related, or catheter-induced. Inflammatory conditions include balanitis xerotica obliterans (BXO), lichen sclerosis, and an inflammatory variant of vitiligo.1,3–6 Procedure-related causes are most commonly associated with transurethral resection of the prostate, which has a reported rate of approximately 2.6%.7 However, we could find no information suggesting that a larger caliber resectoscope was responsible for a greater risk of fossa strictures.
Catheter-induced strictures have been fairly extensively evaluated.8 The incidence of urethral stricture after cardiovascular surgery in the early 1980s prompted much investigation and was thought to be from catheter toxicity and urethral ischemia.9,10 However, these strictures usually affected the anterior urethra, and none solely involved the fossa navicularis. Abdel-Hakim11 and Elhilali12 found some evidence for urethral ischemia in stricture cases utilizing strain gauge plethysmography to determine penile-brachial indexes. In addition, Nacey13 demonstrated a significantly increased incidence of urethral strictures following catheterization with silastic catheters compared with silicone catheters in a controlled randomized prospective study of patients undergoing elective cardiac surgery. Other experimental and clinical studies demonstrated that latex catheters are more toxic than nonlatex catheters.14–18
In a study by Masters and Rice,19 voiding function and urinary symptoms improve as demonstrated by an increase in flow rate and a decrease in IPSS after open radical prostatectomy. Thus, a decrease in flow rate and a higher IPSS suggest stricture or stenosis, or both. In our series, most stricture patients had a markedly decreased flow rate and a higher IPSS, which was confirmed by bougie calibration and cystourethroscopy.
After a review of our first 100 cases, we noted 5 fossa strictures. As noted above, there were essentially no reports of fossa strictures following radical prostatectomy and very little information regarding its cause. Initially, we looked at factors like age, medical diseases, BMI, EBL, operative length, and other things, for a cause. With no obvious factor identified, we evaluated the use of monopolar cautery. We were concerned that electrical current leaving the monopolar tip might potentially be transmitted down the catheter in the urethra and injure the fossa. In response to this potential source of injury, the grounding pad was moved from the upper thigh (ie, adjacent to the penis) to the thorax. However, the fossa strictures continued. Our next thought was that possibly the 22F caliber of the urethral catheter might be responsible. At case number 35, we had switched from our standard 18F catheter to a 22F caliber catheter to avoid inadvertent stapling of the urethra. At case number 166, we switched back. Remarkably, our data suggest and continued experience supports this as the likely cause. A 22F compared with an 18F silastic urethral catheter size used during the intraoperative dissection appears to increase the risk 8-fold. Although no reasonable means exist to definitively prove this, it may be that in addition to catheter size, the combination of an extreme Trendelenburg position and the pneumoperitoneum associated with RLP may be other contributing factors. This might account for the lack of similar stricture experience in the very large volume of reports published on radical prostatectomy and cystectomy.
Spongiofibrosis and fossa stricture formation may also be related to the technique of catheter insertion; however, catheters were inserted by a urologist at the bedside and no difficulty was noted in the operative record. Other findings include the mean time to fossa stricture formation being 50 days (range, 34 to 93). As Table 1 demonstrates, no other obvious differences exist between the 2 groups. Two of 10 strictures (both in the 22F group) subsequently required open reconstruction, whereas 80% resolved with soft dilations continued over a 3-month to 6-month interval following development.
CONCLUSION
An 18F catheter is sufficient to drain the bladder safely. Larger urethral catheter size during the intraoperative dissection in RLP appears to increase the risk for fossa navicularis stricture.
References:
- 1. Armenakas NA, McAninch JW. Management of fossa navicularis stricture. Urol Clin N Am. 2002; 29: 477–484 [DOI] [PubMed] [Google Scholar]
- 2. Ahlering TE, Eichel L, Edwards RA, Lee DI, Skarecky DW. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology. 2004; 64: 1224–1228 [DOI] [PubMed] [Google Scholar]
- 3. Khezri AA, Dounis A, Dunn M. Balanitis xerotica obliterans. Br J Urol. 1979; 51: 229–231 [DOI] [PubMed] [Google Scholar]
- 4. Mall N, Garat JM, Santaularia J, Hernandez J. Urethro-balanitis xerotica obliterans. Eur Urol. 1978; 4: 9–12 [DOI] [PubMed] [Google Scholar]
- 5. Staff WG. Urethral involvement in balanitis xerotica obliterans. Br J Urol. 1970; 47: 234–239 [DOI] [PubMed] [Google Scholar]
- 6. Barbagli G, Palminteri E, Lazzeri M, Turini D. Lichen sclerosis of the male genitalia. Contemp Urol. 2001; 13: 47–50 [Google Scholar]
- 7. Lentz HC, Jr., Mebust WK, Foret JD, Melchior J. Urethral strictures following transurethral prostatectomy: review of 2,223 resections. J Urol. 1977; 117: 194–196 [DOI] [PubMed] [Google Scholar]
- 8. Edwards L, Trott PA. Catheter-induced urethral inflammation. J Urol. 1973; 110: 678–681 [DOI] [PubMed] [Google Scholar]
- 9. Ruutu M, Alfthan O, Heikkinen L, et al. “Epidemic” of acute urethral stricture after open heart surgery. Lancet. 1982; 1: 218. [DOI] [PubMed] [Google Scholar]
- 10. Sutherland PD, Maddern JP, Jose JS, Marshall VR. Urethral stricture after cardiac surgery. Br J Urol. 1983; 55: 413–416 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Hakim A, Hassouna M, Teijeira J, Elhilali MM. Role of urethral ischemia in the development of urethral strictures following cardiovascular surgery: a preliminary report. J Urol. 1984; 131: 1077–1079 [DOI] [PubMed] [Google Scholar]
- 12. Elhilali MM, Hassouna M, Abdel-Hakim A, Teijeira J. Urethral stricture following cardiovascular surgery: role of urethral ischemia. J Urol. 1986; 135: 275–277 [DOI] [PubMed] [Google Scholar]
- 13. Nacey JN, Tulloch AG, Ferguson AF. Catheter induced urethritis: a comparison between silastic and silicone catheters in a prospective clinical trial. Br J Urol. 1985; 57: 325–328 [DOI] [PubMed] [Google Scholar]
- 14. Engelbart RH, Bartone FF, Gardner P, Hutson J. Urethral reaction to catheter materials in dogs. Invest Urol. 1978; 16: 55–56 [PubMed] [Google Scholar]
- 15. Graham DT, Mark GE, Pomeroy AR. Cellular toxicity of urinary catheters. Med J Aust. 1983; 1: 456–459 [DOI] [PubMed] [Google Scholar]
- 16. Keitzer WA, Abreu A, Navarro I, Bernreuter E, Adams JS. Urethral strictures: prevention with plastic indwelling catheters. J Urol. 1968; 99: 187–188 [DOI] [PubMed] [Google Scholar]
- 17. Painter MR, Borski AA, Trevino GS, et al. Urethral reaction to foreign objects. J Urol. 1971; 106: 227–230 [DOI] [PubMed] [Google Scholar]
- 18. Engel RME, Wise HA, Whitaker RH. Otis internal urethrotomy with long term urethral intubation: a comparison of latex and silastic catheters. South Med J. 1972; 65: 55–60 [DOI] [PubMed] [Google Scholar]
- 19. Masters JG, Rice ML. Improvement in urinary symptoms after radical prostatectomy: a prospective evaluation of flow rates and symptom scores. BJU Int. 2003; 91: 795–797 [DOI] [PubMed] [Google Scholar]