Abstract
Background:
Liver cysts have been estimated to occur in 5% of the population. Multiple liver cysts can also be part of the polycystic disease complex. Only symptomatic or complicated cysts need surgery. Traditionally, laparotomy is the procedure of choice. We present our experiences with laparoscopic management of both symptomatic multiple liver cysts and polycystic liver disease.
Methods:
Between 1995 and 2006, we treated 12 patients with large, multiple liver cysts, including 4 cases of polycystic liver disease. Most of the patients were elderly males. The lung and other organs were not involved in any case. Laparoscopic deroofing or radical excision with omentoplasty was successfully performed in these patients.
Results:
Postoperatively, 4 patients had fluid draining through the drainage tube for an average of 10 days. One patient had ascites that resolved spontaneously. Cysts recurred in 5 patients.
Discussion:
There are not many reports in the literature regarding large series of patients, further confirming the rarity of the disease. Liver cysts can occur as a part of polycystic renal and lung disease or isolated to the liver alone. Laparoscopic deroofing is the ideal treatment for nonpolycystic liver disease, and laparoscopic radical excision is ideal for polycystic liver disease. Simple needle aspiration or sclerotherapy is inadequate as recurrence is almost 100%.
Conclusion:
Currently, laparoscopy scores over laparotomy for the treatment of nonparasitic liver cysts as evidenced by this and other studies.
Keywords: Giant liver cyst, Nonparasitic, Polycystic disease, Laparoscopy, Deroofing
INTRODUCTION
The term hepatic cyst usually refers to solitary nonparasitic cysts of the liver, also known as simple cysts. The precise frequency of liver cysts is not known because most do not cause symptoms, but liver cysts have been estimated to occur in 2.5% to 5% of the population.1 If the whole liver is involved, polycystic liver disease (PCLD) is presumed to exist. The cysts are lined with epithelium, and because liver cysts seldom contain bile, the current hypothesis is that they fail to develop normal connections with the biliary tree. The majority of patients afflicted with adult polycystic liver disease are asymptomatic. For those who are symptomatic, a variety of treatment procedures have been proposed. The choice of surgical procedure is related to the severity of the disease and morphology of the cysts within the liver. Most case series in the literature are relatively small, reporting fewer than 50 patients each.2 When the cysts become large and cause symptoms like pain, treatment is warranted. Today, imaging studies often reveal asymptomatic lesions incidentally. Historically, treatment of hepatic cysts has required laparotomy. Anecdotal reports of laparoscopic treatment became common by the mid 1990s. Laparoscopic deroofing of liver cysts was first described in 1991.3 We present our series of 12 patients who underwent laparoscopic surgery for symptomatic polycystic disease of the liver.
METHODS
We have managed 12 patients with large, nonparasitic, simple hepatic cysts laparoscopically, from 1996 to 2006. There were 7 males and 5 females; average age was 65 years (range, 58 to 72). Eight patients had isolated cysts of both lobes of the liver, and the other 4 cases had associated (asymptomatic) polycystic renal disease. The most common symptom was pain and abdominal swelling. All the patients in this series had nonparasitic cysts in both lobes of the liver, the largest measuring 20×14×9 cm in a 72-year-old lady. Routine laboratory investigations including liver function tests and bleeding profile, chest radio-gram, and electrocardiogram were done in all patients. The initial imaging modality was the ultrasonogram in all cases, followed by CT scan. There was no pulmonary involvement in any case. None of the cysts were infected or ruptured. Elective laparoscopic surgery was planned for all patients. For the 4 patients with PCLD, laparoscopic radical excision was performed. The 8 cases with the isolated multiple cysts underwent laparoscopic deroofing of the larger cysts and puncturing of the smaller cysts. Pneumoperitoneum was established by Veress needle, and pressure was maintained at 13 mm Hg. The patient was positioned supine with both legs apart, the head-end raised to 30°. The chief surgeon stood in between the legs, camera surgeon stood to the right of the patient, an assistant and scrub nurse stood on the left side. The monitor was placed on the head-end of the patient, directly opposite the chief surgeon. Four ports were used: (1) a 10 mm port 2 cm above the umbilicus for the laparoscope; (2) a 5 mm port in the left midclavicular line for right-hand working; (3)a5mm port in the right midclavicular line for left-hand working; (4) and a 5 mm port in the epigastrium for liver retraction.
In all the patients, multiple cysts were present in both lobes of the liver. There was always at least one large (dominant) cyst with other smaller cysts. The first step was to introduce the 5 mm left-hand working port directly into the largest cyst and suck out the contents without any spillage (Figure 1). This causes the cyst to collapse (Figure 2). Through the cystotomy opening, a Harmonic ACE scalpel (Ethicon Endosurgery, Cincinnati, OH, USA) was used to cut out the cyst wall (Figure 3). In patients where the cysts were more exophytic, a major portion of the cyst wall was excised. For left lobe lesions, the laparoscope was turned to the left of the falciform ligament and the cysts excised (Figure 4). The smaller cysts were deroofed or punctured. In case the cyst was loculated, all the septae were broken and laid open. We attempted to deroof as many cysts as possible. Complete resection of the cysts was achieved by keeping the dissection close to the cyst wall and was performed in 8 cases. The Harmonic ACE scalpel was used for these resections and found to be very effective. In 3 patients with PCLD, complete resection was abandoned because bleeding was a problem. The greater omentum was packed into the bigger cavities to obliterate dead space and prevent fluid collection. Frozen section examination of tissue bits from the cyst wall revealed no evidence of cystadenoma or carcinoma in any case.
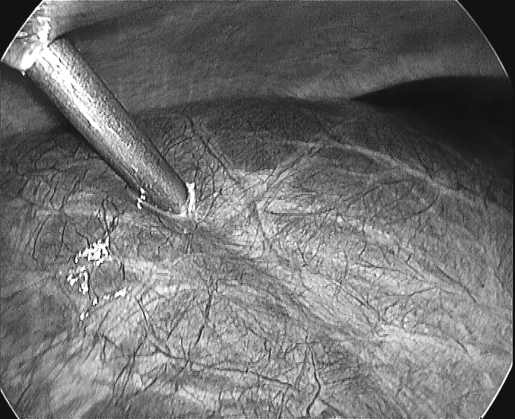
Figure 1.
Puncturing right lobe cyst with direct 5 mm trocar insertion.
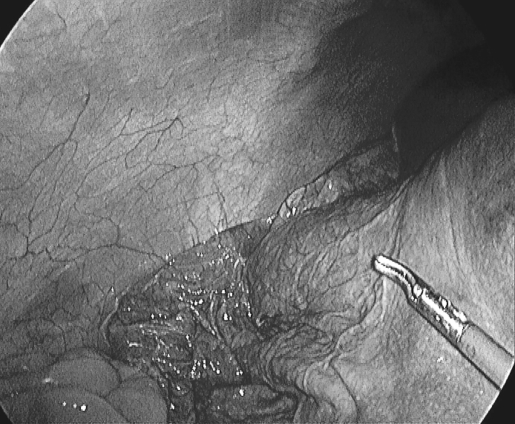
Figure 2.
Cyst collapses after fluid suction.
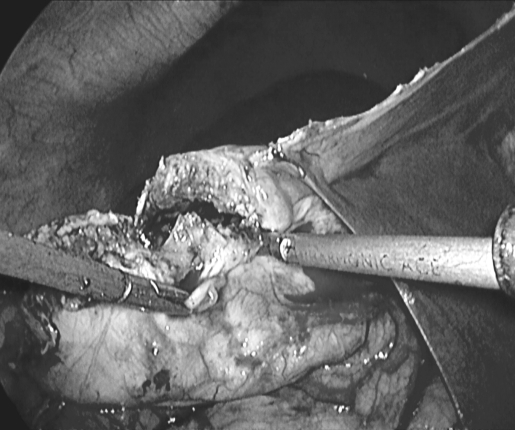
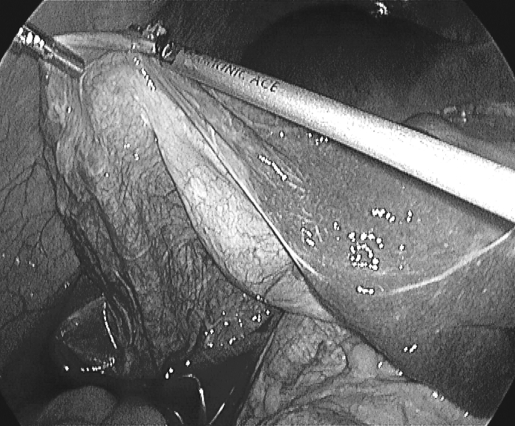
Figure 3.
Cyst being resected.
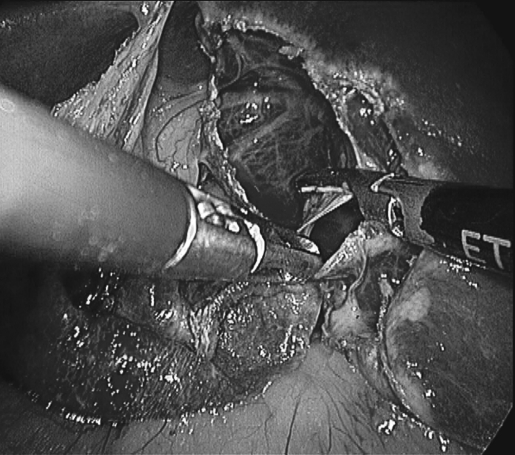
Figure 4.
Deroofing of left lobe cyst; multiple septae seen.
RESULTS
Operating time was 60 minutes to 110 minutes with no significant blood loss. Liquids were started on the same evening as surgery, and a normal diet was started on the first postoperative day (POD). Parenteral analgesics were administered for 1 POD, and thereafter, oral analgesia was sufficient. A drainage tube was placed for the 4 patients who underwent radical cyst excision. Fluid drainage was present for 8 days to 12 days. Between 25 mL and 350 mL of fluid was recorded per patient during the 10 (average) days. These 4 patients were discharged between the third and fifth POD with the drain tube and were advised to empty and measure the fluid daily. The first follow-up was after 5 postoperative days. The drain tube was removed during the first follow-up for all patients. One patient developed ascites, which resolved spontaneously after 30 days. Follow-up was for 12 months to 48 months; 6 patients were followed up with regular ultrasonograms. Cysts recurred in 5 patients between 22 months and 36 months. Six patients were lost to follow-up.
DISCUSSION
Up to 5% of the population has one or more liver cysts; with a sharp rise in incidence with age.4 Women (age 50 to 60 years) are more commonly affected than men are.
Most cysts are thought to arise as a congenital aberration of bile duct development and are termed simple cysts. Several other small cysts within the liver usually accompany the dominant cyst, as we have seen in all the patients in this series. Multiple hepatic cysts are usually considered to be a part of a polycystic disease complex. In our series, 4 patients had associated (asymptomatic) renal polycystic disease. Polycystic liver disease can arise in childhood or adult life, sometimes associated with polycystic kidney disease. Liver cysts only rarely cause hepatic fibrosis and liver failure.5 Occasionally, isolated polycystic liver disease has been reported.6 Although it is easy to attribute symptoms to the presence of a large hepatic cyst, the possibility of coexisting pathology must be excluded. Despite improved imaging techniques, the presence of neoplasia is still difficult to determine before and during surgery.7 Treatment of patients is indicated only in symptomatic patients. Asymptomatic patients do not require therapy because the likelihood of developing complications related to the lesion is lower than the risks associated with treatment.8 No medical therapies for PCLD exist. Cyst aspiration with sclerosis, open or laparoscopic cyst fenestration, combined hepatic resection and fenestration, and liver transplantation are possible treatments. Percutaneous aspiration of symptomatic hepatic cysts is a simple option, but recurrence is invariable, not to mention lifethreatening infection. Several reports detail the efficacy of laparoscopic surgery for both multiple liver cysts and PCLD.9,10 If the gallbladder is closely adherent to the cysts, cholecystectomy may be indicated; one such case occurred in our study where we managed to preserve the gallbladder (Figure 5). Drainage of the operated site is not mandatory in all patients with large liver cysts; it is advisable only if bile leakage is anticipated. Only 4 of our patients needed drainage, out of which none had bile leakage. Ultimately, it is ideal to tailor the procedure in each individual case. In our series, 8 patients underwent laparoscopic radical excision of the cysts to prevent recurrence, and liver resection was not done in any case. In the 5 (41.66%) patients who developed recurrence in our series, only 1 had undergone radical excision. Based on this, we are tempted to think radical excision of cysts could reduce recurrence rates, though more studies are needed to confirm this. Martin et al11 compared the results of laparoscopic deroofing with open deroofing and open resection in 23 patients with simple cysts and 15 patients with polycystic disease. They found that laparoscopic de-roofing of simple cysts was associated with a 20% morbidity rate. The symptomatic recurrence rate was 8% for simple cysts and 71% for polycystic liver disease.11 Recurrence of symptoms was due to growth of the untreated cysts and not to re-expansion of the treated cysts. Lower recurrence rates were seen in a series of large dominant cysts in the anterior segment of the liver. Additional omen-toplasty and oversewing of the margins by a running suture appear to reduce the recurrence rate to between 0% and 14%.12 Liver transplantation has been performed in rare cases, especially when the above-mentioned interventions are not an option.13
Figure 5.
Collapsed cyst seen closely adherent to gall bladder.
CONCLUSION
Laparoscopic radical excision and omentoplasty for all large polycystic lesions and deroofing with simple puncture of all the cysts in cases of non-PCLD are highly effective procedures. Laparoscopy should replace laparotomy, given the morbidity of an upper abdominal incision.
References:
- 1. Vyhnánek J, Duda M, Gryga A, Dlouhý M, Czudek S. Mini-invasive approach to the treatment of liver cysts. Acta Univ Palacki Olomuc Fac Med. 1999;142:123–126 [PubMed] [Google Scholar]
- 2. Martin I, Garden OJ. Laparoscopic radical deroofing of hepatic cysts using the ultrasonic scalpel. Aust N Z J Surg. 1999;69(10):743–744 [DOI] [PubMed] [Google Scholar]
- 3. Z'Graggen K, Metzger A, Klaiber C. Symptomatic simple cysts of the liver: treatment by laparoscopic surgery. Surg Endosc. 1991;5:224–225 [DOI] [PubMed] [Google Scholar]
- 4. Klingler PJ, Gadenstatter M, Schmid T, Bodner E, Schwelberger HG. Treatment of hepatic cysts in the era of laparoscopic surgery. Br J Surg. 1997;84(4):438–444 [PubMed] [Google Scholar]
- 5. Al Kindy NM, Grant CS, Daar AS. Laparoscopic deroofing of hepatic cyst. SQU Journal for Scientific Research: Medical Sciences. 2000;2:125–126 [PMC free article] [PubMed] [Google Scholar]
- 6. Gloor B, Ly Q, Candinas D. Role of laparoscopy in hepatic cyst surgery. Dig Surg. 2002;19(6):494–499 [DOI] [PubMed] [Google Scholar]
- 7. Fiamingo P, Tedeschi U, Veroux M, et al. Laparoscopic treatment of simple hepatic cysts and polycystic liver disease. Surg Endosc. 2003;17(4):623–626 [DOI] [PubMed] [Google Scholar]
- 8. Gigot JF, Legrand M, Hubens G, et al. Laparoscopic treatment of nonparasitic liver cysts: adequate selection of patient and surgical technique. World J Surg. 1996;20:556–561 [DOI] [PubMed] [Google Scholar]
- 9. Diez J, Decoud J, Gutierrez L, Suhl A, Merello J. Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg. 1998;85(l):25–27 [DOI] [PubMed] [Google Scholar]
- 10. Robinson TN, Stiegmann GV, Everson GT. Laparoscopic palliation of polycystic liver disease. Surg Endosc. 2005;19(1):130–132 [DOI] [PubMed] [Google Scholar]
- 11. Martin IJ, Aileen JM, Elspeth CJ, Paul H, James GO. Tailoring the management of nonparasitic liver cysts. Ann Surg. 1998; 228(2):167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emmermann A, Zornig C, Lloyd DM, Peiper M, Bloechle C, Broelsch CE. Laparoscopic treatment of nonparasitic cysts of the liver with omental transposition flap. Surg Endosc. 1997;11:734–736 [DOI] [PubMed] [Google Scholar]
- 13. Andoh H, Sato T, Yasui O, Shibata S, Kurokawa T. Laparoscopic right hemihepatectomy for a case of polycystic liver disease with right predominance. J Hepatobiliary Pancreat Surg. 2004;11(2):116–118 [DOI] [PubMed] [Google Scholar]