Abstract
Background:
Symptomatic subxiphoid incisional hernias present difficult surgical problems, especially in immuno-suppressed cardiac transplant patients. Here, we describe the laparoscopic repair of subxiphoid incisional hernias in patients with a history of cardiac transplantation.
Methods:
Four patients with subxiphoid hernias who had previously undergone heart transplantation were identified from a prospective database. Each underwent a laparoscopic repair with mesh implantation.
Results:
Three patients had a previous open repair. The mean age was 62.5 years, an average of 64.3 months after transplantation. At the time of surgery, all patients were immunosuppressed, and each had a subxiphoid, poststernotomy incisional hernia. Gore dual mesh was used in 2 patients, while Parietex mesh was used in 2. Mean operative time was 122 minutes, and all were completed laparoscopically. The mean length of stay was 6.5 days, and the mean defect size was 286.25 cm2. There was a significant correlation between hernia size and length of stay (P=0.037). Postoperatively, one patient (25%) developed pulmonary edema, and 1 patient (25%) had a prolonged ileus.
Conclusion:
Symptomatic subxiphoid incisional hernias are a challenging surgical problem in patients with a history of sternotomy. Laparoscopic repair is safe and effective in immunosuppressed patients who have previously undergone cardiac transplantation.
Keywords: Subxiphoid hernia, Cardiac transplant, Laparoscopic repair, Immunosuppressed
INTRODUCTION
Subxiphoid incisional hernia is an uncommon complication after median sternotomy. The precise incidence is not known, because most hernias are small, asymptomatic, and do not present to medical attention. Nonetheless, the reported incidence is 1% to 4.2%.1–3 The hernia defect is typically located in the caudad portion of the sternotomy, where the incision enters the abdominal epigastrium, and the defect is relatively protected from intestinal incarceration by the anterior surface of the liver.4 It is thought that lateral traction forces on the abdominal wall predispose this area to herniation.2 Predisposing factors include male sex, obesity, postoperative wound infection, and left heart failure.1 A retrospective study in a tertiary referral center showed that 0.5% of all patients undergoing median sternotomy required operative hernia repair.5 There are no data relating specifically to immunosuppressed patients who had undergone cardiac transplantation.
As with all hernias, operative repair of subxiphoid incisional hernias is indicated for symptoms or incarceration. Due to the difficult anatomical location, results of conventional hernia repair are poor. A recurrence rate of 43% to 80% has been reported with primary midline reapproximation of the fascia.5,6 Recurrence rates appear to be lower with placement of nonresorbable mesh (0% to 32%).3,5,6 Proper placement of mesh requires dissection of the retroxiphoid space to allow a subfascial position and adequate overlap.4
The laparoscopic technique is especially attractive because it allows for excellent subfascial visualization of the epigastrium and edges of the defect, avoidance of the previous incision and minimal tissue trauma. As with all laparoscopic ventral hernia repairs, proper fixation of the mesh depends on the use of both transfascial sutures and helical tacks.7 No tacks or sutures are placed in the cephalad-most portion of the mesh during a subxiphoid incisional hernia repair. This is thought to contribute to a recurrence rate that is higher than laparoscopic repairs of abdominal hernias in other sites. Recurrence rates with the laparoscopic repair can be as high as 30%, but such results are more likely related to a learning curve of the technique.5 Recurrences of 10% or less have been achieved and should become the accepted norm.3,8,9
In addition, this select population of patients is immuno-suppressed further affecting wound healing. The use of prednisone or sirolimus has been especially implicated in postoperative wound problems in the transplant patient population.10,11 Once a hernia develops in these patients, it is possible that continued immunosuppression would render them especially prone to recurrence, making the laparoscopic approach especially attractive.
METHODS
All patients undergoing laparoscopic incisional hernia repair were entered into a prospective longitudinal database at the Yale-New Haven Hospital Minimally Invasive Surgery Center. Between April 2003 and February 2007, four patients who had previously undergone heart transplantation were identified. All 4 patients presented electively with symptomatic incisional hernias. No patients presented with incarceration. All patients presented to the operating room at Yale-New Haven Hospital fully immunosuppressed. All patients were given a single dose of intravenous cephalosporin preoperatively. A cardiac anesthesiologist was involved in each case, and each patient underwent a transesophageal echocardiogram intraoperatively. The peritoneal cavity was insufflated to 15mm Hg with a Veress needle. Adhesiolysis and take down of the falciform ligament was followed by measurement of the hernia defect dimensions and introduction of the mesh. In 2 patients (50%), Parietex mesh (United States Surgical Corporation, Norwalk, CT) was used, and Gore dual mesh (W. L. Gore, Flagstaff, AZ) was used in the other 2 patients.
Mesh fixation was accomplished with the use of 6 to 8 transfascial nonabsorbable sutures in addition to spiral tacks, ensuring at least 3-cm overlap beyond the edge of the hernia defect circumferentially. The cephalad-most portion of the mesh was not secured with either sutures or tacks, but generous retroxiphoid overlap well beyond the edge of the hernia was ensured. All patients were fitted with an abdominal binder at the completion of the operation and recovered in the postanesthesia care unit. Immunosuppression was resumed on the first postoperative day.
In the postoperative period, all patients were followed primarily by the general surgical service in consultation with the CHF/heart transplant medical service. Patients were discharged home after bowel function was resumed, they were tolerating a diet, and pain was well controlled on oral medication.
Correlation between estimated hernia size and postoperative length of stay was done using a paired t test. Significance was determined as P<0.5.
RESULTS
Ninety-seven laparoscopic ventral/incisional hernia repairs were performed between April 2003 and February 2007. Of these, 4 were repairs of poststernotomy subxiphoid epigastric hernias in patients who had previously undergone cardiac transplantation. All 4 patients were male (Table 1), ranging in age from 56 to 67 years old (mean, 62.5). Each was taking one of the following immunosuppression regimens: mycophenolate mofetil and cyclosporine, or mycophenolate mofetil, cyclosporine, and prednisone, or mycophenolate mofetil, sirolimus, and prednisone. The time since the heart transplant at the time of hernia repair ranged from 13 months to 120 months (mean, 64.3).
Table 1.
Patient Characteristics (N = 4)
| Male | 4 |
| Mean Age (yrs) | 62.5 (range 56-67) |
| Mean Time Since Transplant (mos) | 64.3 (range 12-120) |
| Previous Open Repair (%) | 75 |
| Mean Hospital Stay (d) | 6.5 (range 2-15) |
| Mean Defect Size (cm2) | 286.25 (range 144-600) |
Three of the 4 patients (75%) presented with recurrent incisional hernias that were previously repaired by a different surgeon, using the conventional open technique with reapproximation of the fascia in the midline. All patients were admitted electively for repair.
Abdominal insufflation to 15torr was accomplished with the use of a Veress needle. Three 5-mm trocars and one 12-mm trocar were used in each case. The trocars were positioned in the right upper quadrant, left upper quadrant, right midabdomen, and left midabdomen. The mean defect size was 286.25cm2 (range, 144 to 600), and all patients required a moderate adhesiolysis (Figures 1 and 2). No conversions to open were required. Postoperatively, 3 of the 4 patients were recovered in the postanesthesia care unit, while 1 patient was taken to the intensive care unit for recovery during the first night for monitoring. One patient had a minor postoperative complication with delayed return of bowel function (>5 days). One patient had a major postoperative complication. During the first postoperative night, he developed significant pulmonary edema and respiratory distress. No specific cause was identified. The patient responded to diuresis and did not require intubation.
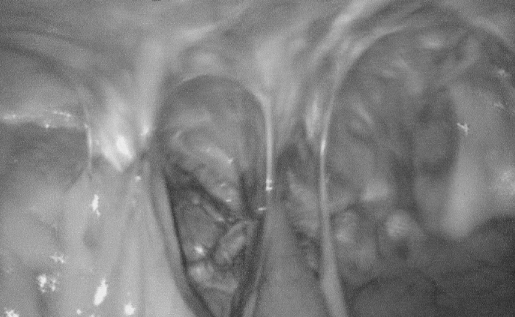
Figure 1.
Intraoperative view of subxiphoid incisional hernia and postoperative adhesions.
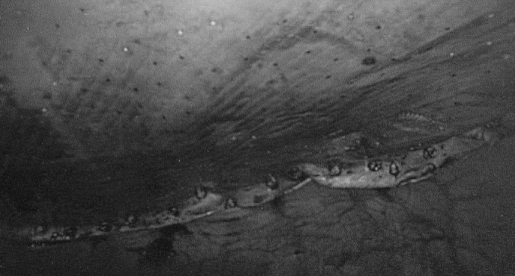
Figure 2.
Intraoperative view of subxiphoid hernia repair with PTFE mesh.
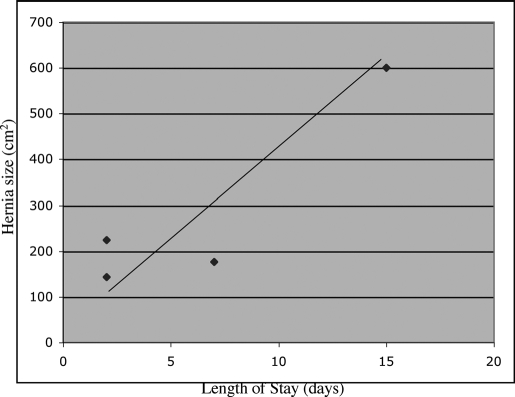
Average length of stay was 6.5 days (range, 2 to 15) and correlated with hernia defect size (Figure 3). A paired t test showed a significant correlation between hernia size and postoperative length of stay (P=0.037). Thirty-day mortality was 0%. Hernia recurrence at 6 months was 0%.
Figure 3.
Linear correlation between size of hernia defect and postoperative length of stay.
DISCUSSION
Among incisional hernias, the poststernotomy subxiphoid incisional hernia is uncommon. Conventional open primary repair of abdominal wall hernias has a high recurrence rate. Subfascial placement of mesh reduces this significantly, and laparoscopy provides an effective, safe, minimally invasive approach.12 The laparoscopic approach to repair of subxiphoid epigastric hernias has been described in few reports in the literature with good success.3,8 Here, we describe the successful laparoscopic repair in a subset of these patients who had undergone heart transplantation. Due to its anatomical position in the epigastrium, this hernia poses a difficult surgical problem. Adequate exposure of the hernia and placement of the mesh usually requires dissection of the falciform ligament. Securing the mesh in this region is especially difficult due to the location proximity of the ribs, diaphragm, and central tendon of the diaphragm. For this reason, we do not secure the cephalad-most portion of the mesh that apposes the central tendon of the diaphragm. Abdominal pressure secures the mesh and adds strength to the repair, and the left lobe of the liver and stomach make this region relatively protected.
Heart transplantation and immunosuppression pose a unique set of challenges in laparoscopic surgery. Despite an increase in survival in heart transplant recipients, these patients develop a host of comorbidities related to the immunosuppressive treatment. Most of these patients over time will develop hypertension, renal dysfunction, coronary artery disease, and diabetes mellitus. Typically, recipients of heart transplants exhibit some degree of diastolic dysfunction as well, while the systolic function may be normal. To safely manage these patients in the operating room, a thorough preoperative evaluation geared mostly towards assessment of cardiovascular function is mandatory. A preoperative transthoracic echocardiogram or even a dobutamine stress echocardiogram should be considered to evaluate the systolic and diastolic function of the transplanted heart as well as cardiovascular reserve. This information will allow the development of a preanesthetic plan that includes fluid management, use of inotropes, the choice of intraoperative monitoring devices, and appropriate adjustment of anesthetic drug doses. At the same time, the surgeon and the anesthesiologist should be prepared to operate at a lower than normal abdominal insufflation pressure according to the cardiovascular reserve of the patient.
The hemodynamic changes incurred with laparoscopy depend on the interaction of several factors including pneumoperitoneum, intraabdominal pressures, carbon dioxide absorption, volume status, and patient position. Typically, the compression of arterial and venous vasculature produces an increase in systemic vascular resistance, mean arterial pressure, filling pressures, and a decrease in preload and cardiac output. These changes are well tolerated by healthy patients. Cardiac transplant recipients, however, manifest an altered cardiac physiology. The transplanted heart lacks autonomic innervation. As a direct consequence, the normal tachycardic and inotropic effect seen during abdominal insufflation is limited. Thus, maintenance of adequate intravascular volume is of extreme importance. However, as mentioned, most of these patients exhibit diastolic dysfunction and are prone to develop pulmonary edema when volume overloaded. The preferred method for assessment of volume status and myocardial function is the transesophageal echocardiogram, which can be performed intraoperatively. If this option is not available then placement of a central line or a pulmonary artery catheter should be considered. Due to a wide variety of hemodynamic responses obtained with abdominal insufflation, a radial artery catheter should be considered in all heart transplant recipients undergoing laparoscopic surgery.
Due to vagal denervation of the transplanted heart, the resting heart rate will probably be 90 to 100 beats per minute, and there will be a loss of bradycardic response to stimuli, such as hypertension or carotid sinus massage. Drugs that normally increase the heart rate by antimuscarinic effects (atropine) will have no effect on the transplanted heart. If an increase in heart rate is desired then medications with direct β-mimetic effect should be used (isoproterenol or epinephrine).
Other considerations should be given to the heart transplant recipient. Since these patients are immunosup-pressed and prone to infection, timely administration of preoperative antibiotic is mandatory. At the same time, placement of central lines should be performed under strict sterile conditions. If the patient is still on glucocorticoids, an intraoperative stress dose of steroids should be considered.
In the postoperative period, the patient should be placed in a monitored setting (telemetry or intensive care unit). Conditions that increase myocardial oxygen demand, such as pain, shivering, and anemia, should be treated promptly. Caution should be exhibited with volume administration to prevent hypovolemia as well as the development of pulmonary edema, as was the case with one of our patients.
The outcome of this repair is excellent, and the result can be life changing. One of our patients was a bilateral leg amputee who could not exhibit sufficient mobility to be fitted for prostheses, due to his very large incisional hernia. Following hernia repair, he was successfully fitted with prostheses and is now ambulatory.
Due to the physiologic complexity of immunosuppressed cardiac transplant patients, we believe it is important to involve a dedicated cardiac anesthesiologist in these cases. With this knowledge, the laparoscopic repair of a poststernotomy incisional hernia in cardiac transplant patients can be performed safely and effectively. This repair is technically challenging, yet it can be performed with little morbidity and mortality.
We also found that the duration of the postoperative length of stay was directly related to the size of the initial hernia defect. This finding mirrored our results for all our other laparoscopic incisional hernia repairs. A larger defect likely reflects the amount of adhesiolysis that is required for repair and the potential extent of fluid shifts. Both these factors can contribute to a prolonged postoperative course. Although we did not treat the wounds in these immunosuppressed patients any differently than in other patients, we saw no wound complications.
CONCLUSION
Symptomatic subxiphoid incisional hernias are a challenging surgical problem in patients with a history of sternotomy. Laparoscopic repair is feasible and safe in immuno-suppressed patients who had previously undergone cardiac transplant.
Contributor Information
Dan Eisenberg, Department of Surgery, Palo Alto Veterans' Affairs Health Care System, Stanford University School of Medicine, Palo Alto, California, USA..
Wanda M. Popescu, Department of Anesthesiology, Yale University School of Medicine, Yale-New Haven Hospital, New Haven, Connecticut, USA..
Andrew J. Duffy, Department of Surgery, Yale University School of Medicine, Yale-New Haven Hospital, New Haven, Connecticut USA..
Robert L. Bell, Department of Surgery, Yale University School of Medicine, Yale-New Haven Hospital, New Haven, Connecticut USA..
References:
- 1.Davidson BR, Bailey JS. Incisional herniae following median sternotomy incisions: their incidence and aetiology. Br J Surg. 1986;73:995–996 [DOI] [PubMed] [Google Scholar]
- 2.Losanoff JE, Basson MD, Laker S, Weiner M, Webber JD, Gruber SA. Subxiphoid incisional hernias after median sternotomy. Hernia. 2007;11:473–479 Epub 2007 Jul 18 [DOI] [PubMed] [Google Scholar]
- 3.Landau O, Raziel A, Matz A, Kyzer S, Haruzi I. Laparoscopic repair of poststernotomy subxiphoid epigastric hernia. Surg Endosc. 2001;15:1313–1314 [DOI] [PubMed] [Google Scholar]
- 4.Conze J, Prescher A, Kisielinski K, Klinge U, Schumpelick V. Technical consideration for subxiphoidal incisional hernia repair. Hernia. 2005;9:84–87 [DOI] [PubMed] [Google Scholar]
- 5.Mackey RA, Brody FJ, Berber E, Chand B, Henderson JM. Subxiphoid incisional hernias after median sternotomy. J Am Coll Surg. 2005;201:71–76 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, Starling JR. Repair of subxiphoid incisional hernia with Marlex mesh after median sternotomy. Arch Surg. 1985;120:1270–1271 [DOI] [PubMed] [Google Scholar]
- 7.van't Riet M, de Vos van Steenwijk PJ, Kleinrensink GJ, Steyerberg EW, Bonjer HJ. Tensile strength of mesh fixation methods in laparoscopic incisional hernia repair. Surg Endosc. 2002;16:1713–1716 [DOI] [PubMed] [Google Scholar]
- 8.Muscarella P, Needleman BJ, Goldstein AH, Steinberg SM. Laparoscopic repair of a subxiphoid incisional hernia following median sternotomy. Surg Rounds. 2000;23:605–611 [Google Scholar]
- 9.Awad ZT, Miedema B. Subxiphoid incisional hernias after median sternotomy. J Am Coll Surg. 2006;202:386–387 [DOI] [PubMed] [Google Scholar]
- 10.Valente JF, Kricik D, Weigel K, et al. Comparison of sirolimus vs. mycophenolate mofetil on surgical complications and wound healing in adult kidney transplantation. Am J Transplant. 2003;3:1128–1134 [DOI] [PubMed] [Google Scholar]
- 11.Janssen H, Lange R, Erhard J, Malago M, Eigler FW, Broelsch CE. Causative factors, surgical treatment and outcome of incisional hernia after liver transplantation. Br J Surg. 2002;89:1049–1054 [DOI] [PubMed] [Google Scholar]
- 12.Heniford BT, Ramshaw BJ. Laparoscopic ventral hernia repair: a report of 100 consecutive cases. Surg Endosc. 2000;14:419–423 [DOI] [PubMed] [Google Scholar]