Abstract
Background:
The purpose of this pilot study was to evaluate the impact of RealHand instruments on laparoscopic-assisted vaginal hysterectomy (LAVH) for the treatment of stage I uterine cancer.
Methods:
This was a single-center, nonrandomized, consecutive patient pilot study. Patient status was evaluated in terms of operative morbidity, length of surgery, anesthesia time, body mass index (BMI), estimated blood loss, uterine weight, and hospital stay.
Results:
In the group of 10 patients, mean operative time was 1.7 hours, and anesthesia time was 2.3 hours. Mean estimated blood loss was 70mL, and patient hospital stay was 31.8 hours. No intra- or postoperative complications occurred. Blood loss, anesthesia time, BMI, and uterine weight were significant predictors of operative time. In one patient, LAVH using the RealHand instruments was canceled because of deep pelvic visualization difficulties, resulting in a conversion to laparotomy.
Conclusion:
We present the first reported individual physician LAVH experience using RealHand instruments for the treatment of clinical stage I uterine cancer. The reported operative time, reasonable patient complication rates, and acceptable postoperative stay suggest that these innovative surgical instruments may have significant promise in the treatment of patients diagnosed with this gynecologic disease.
Keywords: RealHand, LAVH, Uterine malignancy, Complications
INTRODUCTION
Hysterectomy is the second most common surgical procedure performed in the United States, with nearly 600,000 cases reported annually.1 Laparoscopic hysterectomy was initially reported in 19892 and is frequently indicated for the treatment of uterine leiomyoma, endometriosis, and uterine prolapse.3 Laparoscopic-assisted vaginal hysterectomy (LAVH) involves removal of the uterus and potentially the fallopian tubes, ovaries, or both, through the vagina. The LAVH procedure is associated with improved patient outcomes, reduced morbidity, and shorter hospital stay compared with conventional abdominal surgery,4,5 although some controversy surrounds the surgical cost and intraoperative complications.6,7
Despite the extensive range of indications for LAVH, physicians may be constrained by the reduced dexterity and surgical accessibility associated with this approach.8,9 For example, conventional laparoscopic devices permit only up/down and lateral motions potentially impairing access to uterine vessels. Because inadequate articulation significantly restricts a surgeon's visibility and access, determining the primary surgical route may also be confounded.10 Consequently, innovative technology is being evaluated in an attempt to significantly enhance a physician's mobility and range of motion for the surgical treatment of gynecologic disorders.11
RealHand (Novare Surgical Systems, Cupertino, CA) instruments are a novel articulating approach to performing minimally invasive LAVH. They offer dynamic articulation with mirrored hand movements (eg, up/down, left/right, rotation, in/out activation) that extend beyond normal manipulation within the contained surgical field, thereby allowing surgeons to utilize the full spectrum of accessibility during laparoscopic operations.
These novel single-use instruments permit full mobility and tactile feedback within the abdominal cavity, thus precluding the excessive cost and setup time that is currently associated with robotic surgical systems. We present herein the first reported experience evaluating RealHand instruments for the treatment of clinical stage I uterine cancer.
METHODS
From May 2007 until December 2007, 12 consecutive stage I endometrial carcinoma patients were scheduled to undergo LAVH, bilateral salpingo-oophorectomy, and bilateral pelvic lymphadenectomy by an individual gynecologic oncologist. However, 2 patients were excluded from the study because the Novare surgical representative was not present at the surgery, and thus a conventional LAVH was performed. These patients' data were excluded from the statistical analyses.
In all patients, uterine malignancy was confirmed via endometrial biopsy, and there were no palpable indications to initially perform a laparotomy. The patients' mean age was 62.4 years (95% CI=54.43 to 70.37), height was 63.6 inches (95% CI=62.08 to 65.12), and weight was 171.48 pounds (95% CI=137.13 to 205.83). Patient mean BMI was 29.7 (95% CI=24.11 to 35.25). Four patients were obese, one was overweight, and 5 were normal weight.12 An institutional review board approved this study. Patient consent was obtained prior to any data collection, and none of the study participants had a prior history of abdominal surgery.
Preoperative evaluation excluded malignant and premalignant diagnoses in all patients. The participants' primary presenting symptoms were pelvic pain, bleeding, and vaginal discharge. Table 1 exhibits the patients' final pathologic diagnosis. Patients were all classified as P2 according to American Society of Anesthesiologists standards. The following data were collected for the completion of this study: patient characteristics, blood loss, length of hospital stay, surgery and anesthesia time, uterine weight, intraoperative and postoperative complications. Estimated blood loss was calculated by using total evacuated fluid (blood + irrigation) less total irrigation fluid used.
Table 1.
Patients' Pathologic Diagnosis (N =10)
| Patient Histology | Stage | Grade | |
|---|---|---|---|
| 1 | Endometrial adenocarcinoma | 1b | 1 |
| 2 | Endometrial adenocarcinoma | 1b | 2 |
| 3 | Endometrial adenocarcinoma w/clear cell component | 1a | 3 |
| 4 | Endometrial adenocarcinoma | 1b | 2 |
| 5 | Endometrial adenocarcinoma w/mucinous component | 1a | 2 |
| 6 | Endometrial adenocarcinoma | 3a | 1 |
| 7 | Endometrial adenocarcinoma w/endometrioid component | 1b | 2 |
| 8 | Endometrial adenocarcinoma | 1b | 1 |
| 9 | Endometrial adenocarcinoma | 1a | 1 |
| 10 | Endometrial adenocarcinoma | 1a | 1 |
Instrument
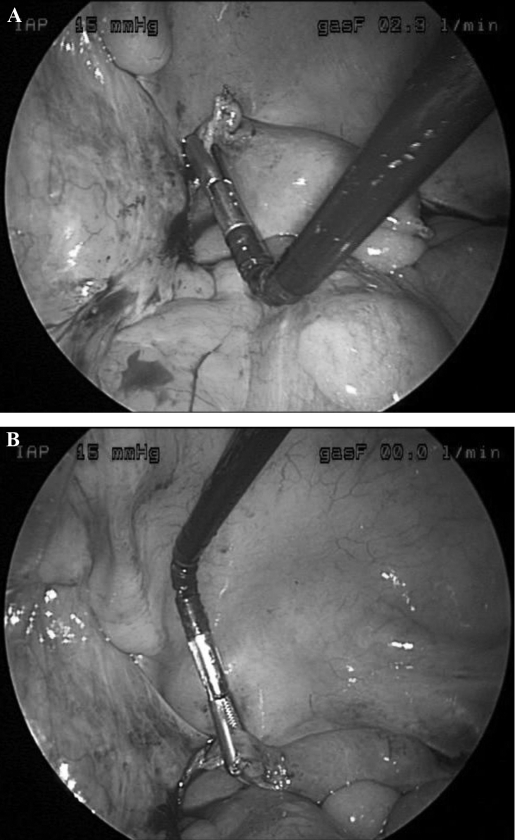
RealHand instruments permit full range of motion and were developed for minimally invasive laparoscopic surgery. The instruments comprise a cautery, grasper, dissector, and a ThermaSeal (seals and separates tissue). The technology is configured to accommodate a surgeon's hand direction and movement, while simultaneously providing concurrent tactile response. The instruments facilitate active manipulation and task completion, regardless of position (ie, over, under, or around structures). Therefore, when a surgeon's hand moves to a location, the instrument's tip follows suit and facilitates access to more remote structures. Because the instruments offer 7 degrees of freedom during movement, a physician is able to perform more complex procedures that are normally precluded by traditional rigid instruments (Figure 1).
Figure 1.
RealHand grasper manipulating uterine cornu.
Procedure
All patients were initially taken to the operating room and placed in a supine position. They were then situated in a dorsal lithotomy position and placed in Allen stirrups, wherein they were subsequently prepped and draped. A panel of anesthesiologists elected to use an appropriate spectrum of anesthetics to induce general anesthesia, none of which resulted in emesis, nausea, or prolonged patient hospital stay. Following general anesthesia, a Foley catheter was inserted transurethrally. Prophylactic antibiotics were administered, consistent with recommendations for abdominal hysterectomy.13
Twelve-millimeter ports were placed umbilically and suprapubically. A 5-mm port was then placed in the right lower quadrant. The pelvis and abdomen were explored, with the findings and a single washing submitted for cytology. Using Harmonic scissors, the surgeon divided the infundibulo-pelvic ligament, psoas peritoneum, round ligaments, and vesico-uterine peritoneum. Subsequently, a bilateral pelvic lymphadenectomy was performed, the boundaries of which comprised the genito-femoral nerves laterally, the deep circumflex and iliac veins distally, the obturator nerves posteriorly, and the umbilical arteries medially. All lymphatic tissue within these aforesaid boundaries was removed and submitted for analysis as pelvic lymph nodes.
The vaginal portion of the procedure was initiated by infiltrating the circum-cervical vagina with 0.25% Marcaine with epinephrine and then making a circum-cervical vaginal incision. Anterior and posterior cul-de-sacs were entered, and then by using a series of Zeppelin clamps, pedicles of the utero-sacral ligaments, uterine vessels, and parametria were developed, divided, and ligated with 2–0 Vicryl until the entire specimen (ie, the uterus, cervix, and adnexa) was removed. The vagina was then closed with a baseball stitch via 2–0 Vicryl.
The pneumoperitoneum was reintroduced, and the pelvis was irrigated with saline. All instruments and trocars were then removed. The 12-mm port sites and fascia were then closed with 2–0 Vicryl and 3–0 Monocryl subcuticular sutures, respectively. All sites were reapproximated with Dermabond, and the patients were then taken to the recovery room.
Patients ambulated on the day of surgery. A normal diet was initiated, and the bladder catheter was removed on the first postoperative day. Patients were then discharged from the surgery center after an overnight stay with oral pain medication.
RESULTS
A statistical analysis (MedCalc; version 9.1) was performed to evaluate the data from the 10 patients who were the subject of this study. Mean operative (surgery and setup) time was 1.7 hours (95% CI=1.45 to 1.86), and anesthesia time was 2.3 hours (95% CI=2.05 to 2.48). Mean uterine weight was 83.4 grams (95% CI=55.66 to 111.14). Mean estimated blood loss was 70 mL (95% CI=35.45 to 104.55), and hospital stay was 31.8 hours (95% CI=29.62 to 34.07).
No major or minor intra- or postoperative complications were encountered with the patient surgeries. Blood loss was reasonable, and no significant complications or trips to the emergency room occurred. However, in one of the surgeries, a patient had a large classified subserosal leiomyoma that resulted in deep pelvic visualization difficulties. She experienced both a spontaneous drop in her oxygen saturation and compromised ventilatory effort. Consequently, the procedure was converted to laparotomy.
In a multivariate regression analysis, we examined the prognostic impact of blood loss, BMI, and uterine weight on the length of surgery. The model was significant at predicting length of surgery (R2=0.90; P<0.011). We further explored these predictor variables to determine their relationship with length of surgery. The evaluation revealed significant relationships between surgery length and BMI (r=0.80; P=0.005), blood loss (r=0.70; P=0.02), anesthesia time (r=0.92; P=0.0001), and uterine weight (r=0.71; P=0.02).
We also investigated the prognostic impact of surgery time, uterine weight, blood loss, and BMI on patient time in the facility. None of the aforementioned variables significantly impacted patient time in the facility (R2=0.74; P=0.22).
DISCUSSION
Laparoscopic-assisted vaginal hysterectomy is primarily indicated for patients with endometriosis, adhesions, and fibroids.3 Furthermore, the procedure is associated with low morbidity rates, favorable recovery time, and reduced pain in comparison with both abdominal hysterectomy and conventional vaginal hysterectomy.4,5,9 However, LAVH can be a protracted and complicated surgery because of reduced dexterity and surgical accessibility during the operative course.10
The RealHand surgical instruments were developed to provide greater surgical dexterity and control when operating in close proximity to critical structures and vasculature. The capacity for increased range of motion was also designed to potentially facilitate and enhance intra- and postoperative surgical outcomes. One may argue that the dexterity and control of RealHand offer a better solution to lymphadenectomy, primarily in comparison to LAVH. In particular, the articulation appears to allow for a more accurate targeting of nodes.
In terms of cost, we contend that the RealHand is financially justified because only one instrument per case is used, in contrast with an entire set. Moreover, the RealHand devices may be substituted for another disposable instrument that the surgeon typically uses (eg, disposable scissors). While we did not conduct a cost analysis, we suspect that the RealHand instruments warrant additional fiscal consideration because the articulating laparoscopic capacity increases surgical dexterity without the additional training and expenses inherent in performing robotic surgery.
In the current study, we present the first surgical experience of an individual gynecologic oncologist who performed all operations with the RealHand surgical instruments at a single facility. Patient operative time was within normal limits (1.7 hours) but distinctly less than the 3.7 hours reported by Frigerio et al.14 Initially, we considered further analyzing patient operative time to discern a possible learning curve, but we recognize that the results would be inconclusive due to the small patient population. We also acknowledge the difficulty in comparing the impact of innovative surgical technology because the data are often confounded by multiple physician involvement, in-patient care, and institutional variability.15,16
Patient blood loss (70mL) was a significant prognostic indicator of increased surgery time. However, our rate was similar to that reported in prior LAVH studies.14,17 We suggest that the patients' uterine weight may have resulted in longer surgery times, although additional LAVH outcomes studies have not reported analogous findings.4,15,18 Increased BMI appeared to affect operative time, which was expected considering that 4 of the patients were obese. Therefore, because obesity may be associated with more complicated surgeries, corresponding protracted operative times should be anticipated.19
Patient length of hospital stay (31.8 hours) was similar to that reported by Gemignani et al,17 but dramatically less than the 64.8 or 96 hours reported in previous studies.14,20 Bladder, urinary tract, and bowel injuries are frequently reported complications in LAVH outcome studies,7,21 although none of these conditions manifested themselves in the present series. Despite the reasonable complication rate, one patient was converted to laparotomy due to deep pelvic visualization difficulties.
We recognize that the limited number of patients, absence of a control population, and nonrandomized nature of this single-institutional study preclude any definitive conclusions or comparisons with previously reported LAVH studies. Nevertheless, the results from this study indicate that these innovative instruments may have significant promise in the treatment of stage I uterine cancer and potentially for higher stage uterine cancer procedures. Additional investigation comparing these surgical devices with standard instruments in a larger randomized study involving different uterine cancer populations is warranted.
Footnotes
This study was sponsored by Novare Surgical Systems, Inc. and The Women's Cancer Research Foundation.
Contributor Information
Mark A. Rettenmaier, Gynecologic Oncology Associates, Hoag Memorial Hospital Cancer Center, Newport Beach, California, USA..
Katrina Lopez, Gynecologic Oncology Associates, Hoag Memorial Hospital Cancer Center, Newport Beach, California, USA..
Cheri L. Graham, Gynecologic Oncology Associates, Hoag Memorial Hospital Cancer Center, Newport Beach, California, USA..
John V. Brown, Gynecologic Oncology Associates, Hoag Memorial Hospital Cancer Center, Newport Beach, California, USA..
Cameron R. John, Utah Valley State College, Department of Behavioral Sciences..
John P. Micha, Gynecologic Oncology Associates, Hoag Memorial Hospital Cancer Center, Newport Beach, California, USA..
References:
- 1.Reich H, Decaprio J, McGlynn F. Laparoscopic hysterectomy. J Gynecol Surg. 1989;5:213–216 [Google Scholar]
- 2.O'Hanlan KA, Lopez L, Dibble SL. Total laparoscopic hysterectomy: body mass index and outcomes. Obstet Gynecol. 2003;102:1384–1392 [DOI] [PubMed] [Google Scholar]
- 3.Mettler L, Ahmed-Ebbiary N, Schollmeyer T. Laparoscopic hysterectomy: challenges and limitations. Minim Invasive Ther Allied Technol. 2005;14:145–159 [DOI] [PubMed] [Google Scholar]
- 4.Jaturasrivilai P. A comparative study between laparoscopically assisted vaginal hysterectomy and abdominal hysterectomy. J Med Assoc Thai. 2007;90:837–843 [PubMed] [Google Scholar]
- 5.Kalogiannidis I, Lambrechts S, Amant F, Neven P, Van Gorp T, Vergote I. Laparoscopy-assisted vaginal hysterectomy compared with abdominal hysterectomy in clinical stage I endometrial cancer: safety, recurrence, and long-term outcome. Am J Obstet Gynecol. 2007;196:248. [DOI] [PubMed] [Google Scholar]
- 6.Hidlebaugh D, O'Mara P, Conboy E. Clinical and financial analyses of laparoscopically assisted vaginal hysterectomy versus abdominal hysterectomy. J Am Assoc Gynecol Laparosc. 1994;1:357–361 [DOI] [PubMed] [Google Scholar]
- 7.Shen CC, Wu MP, Kung FT, et al. Major complications associated with laparoscopic-assisted vaginal hysterectomy: ten-year experience. J Am Assoc Gynecol Laparosc. 2003;10:147–153 [DOI] [PubMed] [Google Scholar]
- 8.Sizzi O, Paparella P, Bonito C, Paparella R, Rossetti A. Laparoscopic assistance after vaginal hysterectomy and unsuccessful access to the ovaries or failed uterine mobilization: changing trends. JSLS. 2004;8:339–346 [PMC free article] [PubMed] [Google Scholar]
- 9.Chang WC, Torng PL, Huang SC, et al. Laparoscopic-assisted vaginal hysterectomy with uterine artery ligation through retrograde umbilical ligament tracking. J Minim Invasive Gynecol. 2005;12:336–342 [DOI] [PubMed] [Google Scholar]
- 10.David-Montefiore E, Rouzier R, Chapron C, Daraï E. Collegiale d'Obstetrique et Gynecologie de Paris-Ile de France: Surgical routes and complications of hysterectomy for benign disorders: a prospective observational study in French university hospitals. Hum Reprod. 2007;22:260–265 [DOI] [PubMed] [Google Scholar]
- 11.Hart R, Doherty DA, Karthigasu K, Garry R. The value of virtual reality-simulator training in the development of laparoscopic surgical skills. J Minim Invasive Gynecol. 2006;13:126–133 [DOI] [PubMed] [Google Scholar]
- 12.Bulik CM, Wade TD, Heath AC, et al. Relating body mass index to figural stimuli: population-based normative data for Caucasians. Int J Obes. 2001;25:1517–1524 [DOI] [PubMed] [Google Scholar]
- 13.DiLuigi AJ, Peipert JF, Weitzen S, Jamshidi RM. Prophylactic antibiotic administration prior to hysterectomy: a quality improvement initiative. J Reprod Med. 2004;49:949–954 [PubMed] [Google Scholar]
- 14.Frigerio L, Gallo A, Ghezzi F, Trezzi G, Lussana M, Franchi M. Laparoscopic-assisted vaginal hysterectomy versus abdominal hysterectomy in endometrial cancer. Int J Gynaecol Obstet. 2006;93:209–213 [DOI] [PubMed] [Google Scholar]
- 15.McClellan SN, Hamilton B, Rettenmaier MA, et al. Individual physician experience with laparoscopic supracervical hysterectomy in a single outpatient setting. Surg Innov. 2007;14:102–106 [DOI] [PubMed] [Google Scholar]
- 16.Karaman Y, Bingol B, Günenç Z. Prevention of complications in laparoscopic hysterectomy: experience with 1120 cases performed by a single surgeon. J Minim Invasive Gynecol. 2007;14:78–84 [DOI] [PubMed] [Google Scholar]
- 17.Gemignani ML, Curtin JP, Zelmanovich J, Patel DA, Venkatraman E, Barakat RR. Laparoscopic-assisted vaginal hysterectomy for endometrial cancer: clinical outcomes and hospital charges. Gynecol Oncol. 1999;73:5–11 [DOI] [PubMed] [Google Scholar]
- 18.Torng PL, Chang WC, Hwang JS, et al. Health-related quality of life after laparoscopically assisted vaginal hysterectomy: is uterine weight a major factor? Qual Life Res. 2007;16:227–237 [DOI] [PubMed] [Google Scholar]
- 19.Gong EM, Orvieto MA, Lyon MB, Lucioni A, Gerber GS, Shalhav AL. Analysis of impact of body mass index on outcomes of laparoscopic renal surgery. Urology. 2007;69:38–43 [DOI] [PubMed] [Google Scholar]
- 20.Tollund L, Hansen B, Kjer JJ. Laparoscopic-assisted vaginal vs. abdominal surgery in patients with endometrial cancer stage 1. Acta Obstet Gynecol Scand. 2006;85:1138–1141 [DOI] [PubMed] [Google Scholar]
- 21.Soong YK, Yu HT, Wang CJ, Lee CL, Huang HY. Urinary tract injury in laparoscopic-assisted vaginal hysterectomy. J Minim Invasive Gynecol. 2007;14:600–605 [DOI] [PubMed] [Google Scholar]