Abstract
Background:
The use of robotic assistance in adult genitourinary surgery has been successful in many operations, leading surgeons to test its use in other applications as well.
Methods:
Based on our use during prostatectomy, we have applied robotic surgery to complex distal ureteral surgeries since 2004 with successful outcomes.
Results:
A series of 11 patients who underwent robot-assisted laparoscopic distal ureteral surgery is presented. These surgeries include distal ureterectomy for ureteral cancer with reimplantation, as well as reimplantation with and without Boari flap or psoas hitch for benign conditions.
Conclusions:
Robot-assisted laparoscopic surgery can be successfully applied to patients requiring distal ureteral surgery. Maintenance of the principles of open surgery is paramount.
Keywords: Robotics, Laparoscopic surgery, Ureter, Reimplantation
INTRODUCTION
Since the beginning of the 21st century, the da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA) has been used most commonly in adult urologic surgery for prostatectomy. With expanding experience in this procedure, we have looked at other procedures that may benefit from robot-assisted laparoscopic surgery.
Improved 3-dimensional visualization, precise translation of hand movements, increased degrees of freedom and wristed motions make the robotic technique superior to conventional laparoscopy or traditional open surgery.1 The improved ability to perform intracorporeal suturing makes ureteral surgery a logical extension based on experience with anastomoses in prostatectomy. We present our case series to demonstrate the breadth of distal ureteral surgery that can be accomplished using robotic assistance.
CASE REPORT
The 11 patients who underwent robot-assisted laparoscopic ureteral surgery from 2004 through 2008 are summarized in Table 1. None of these cases required conversion to the laparoscopic or open approach.
Table 1.
Men Who Underwent Robot-Assisted Laparoscopic Distal Ureteral Surgery
| Age (yr)/Sex | Diagnosis | Surgery | OR Time (min) | Cysto Time (min) | Robot Time (min) | EBL (mL) | LOS (d) | Complications | Pathology | Follow-up (mos) |
|---|---|---|---|---|---|---|---|---|---|---|
| 65/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, ureteral reimplant, pelvic lymph node dissection | 207 | 5 | 175 | 200 | 2 | None | Low-grade transitional cell cancer, TAN0M0, grade 1 | 53 |
| 54/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, pelvic lymph node dissection | 170 | 6 | 140 | 50 | 2 | None | Negative for residual malignancy | 46 |
| 81/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, pelvic lymph node dissection | 175 | 5 | 148 | 0 | 2 | None | T2N0M0, high-grade papillary urothelial carcinoma | 39 |
| 60/M | Hutch bladder diverticulum | Robot-assisted laparoscopic diverticulectomy, ureteral reimplant | 240 | 20 | 210 | 25 | 2 | None | Bladder diverticulum, negative for malignancy | 36 |
| 60/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, reimplant, pelvic lymph node dissection | 202 | 5 | 168 | 300 | 1 | None | T2N1M0, high-grade, invasive papillary carcinoma; positive nodal disease | 19 |
| 75/F | Recurrent right ureteral strictures | Robot-assisted laparoscopic Boari flap, ureteral reimplant | 172 | 0 | 150 | 0 | 2 | External iliac vein injury repaired robotically | None | 12 |
| 70/M | Hydronephrosis with distal ureteral obstruction secondary to lymphoma (no evidence of disease); multiple prior ureteral manipulations | Robot-assisted psoas hitch and ureteral reimplant | 189 | 0 | 157 | 0 | 1 | None | None | 8 |
| 38/F | Ureteral damage during hysterectomy | Robot-assisted psoas hitch and ureteral reimplant | 145 | 0 | 110 | 0 | 1 | None | None | 6 |
| 76/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, Boari flap/ureteral reimplant, pelvic lymph node dissection | 224 | 11 | 188 | 200 | 5 | Ileus; no intervention | TAN0M0 high-grade transitional cell cancer | 4 |
| 71/M | Bladder diverticulum with transitional cell cancer | Robot-assisted laparoscopic diverticulectomy, ureteral reimplant | 162 | 30 | 112 | 25 | 4 | None | T3AN0M0 high-grade transitional cell cancer | 2 |
| 67/M | Ureteral cancer | Robot-assisted laparoscopic distal ureterectomy, psoas hitch reimplant, pelvic lymph node dissection | 197 | 10 | 171 | 100 | 5 | Hematuria controlled with fulguration | TAN0M0 low-grade transitional cell cancer | 1 |
Preoperative discussion with these patients includes an explanation of open surgery, laparoscopic surgery, and robotic surgery. The benefits and risks of these approaches are discussed with the patients, including the possibility of conversion to open surgery. When appropriate, patients are consented for possible contingencies, such as psoas hitch, Boari flap, and ileal ureter. Patients complete a bowel preparation, including clear liquids the day before surgery, magnesium citrate on the afternoon before surgery, and an enema before going to bed.
All patients are positioned for general anesthesia in a dorsal lithotomy, steep Trendelenburg position using shoulder bolsters. Prophylactic antibiotics, Venodyne compression boots (Microtek Medical, Columbus, Mississippi), and prophylactic subcutaneous heparin are administered. For distal ureteral tumors, a double-J stent is placed cystoscopically in the affected side, and an open-ended catheter is placed contralaterally to prevent injury; in benign cases, the stent is placed via a laparoscopic port. In cases involving a bladder diverticulum, an angiography catheter is placed within the diverticulum to provide distension.
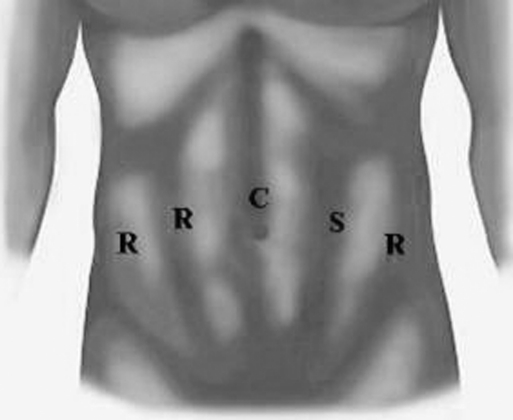
Peritoneal access is achieved with the Hasson technique or Veress needle, depending on the patient's surgical history. The robot is docked immediately, and port placement is shown in Figure 1. Our institution has 2 da Vinci-S systems (4 arms) introduced in 2006, and 1 da Vinci system (3 arms). For a 3-arm robotic system, the most lateral robotic port on the patient's right side is not used.
Figure 1.
Placement of ports for robot-assisted laparoscopic distal ureteral surgery using the da Vinci-S 4-arm system. For a 3-arm system, the most lateral robotic port on the patient's right side would be an assistant port of any trocar size. C = camera port (12 mm), R = robotic ports (8 mm), S = suction/assistant port (12 mm)
During cases involving malignancy, careful attention is paid to maintaining oncologic principles against tumor seeding. All dissection is performed using the robot. The wristed instruments of the robot facilitate dissection in small spaces as well as intracorporeal suturing and knot tying. Robotic Pott's scissors aid in precise spatulation of the ureter, which then facilitates the anastomosis. No attempt is made at performing a nonrefluxing reimplant. A 7F ureteral stent of appropriate length is placed. Jackson-Pratt drains are left in the pelvis.
Total operating room time, which we define as the start of cystoscopy if performed or skin incision until last skin stitch, ranged from 145 minutes to 240 minutes with a median of 189 minutes, mean 189 minutes. Cystoscopy time ranged from 0 minutes to 30 minutes with a median of 5 minutes. The robotic portion, which we define as time spent at the console by the surgeon, ranged from 110 minutes to 210 minutes with a median of 157 minutes, mean 157 minutes.
Drain fluid is sent to the laboratory for analysis of creatinine levels on postoperative day #1 for every patient; if this fluid is serous, the drains are removed prior to discharge. No patients in this series had a urine leak.
Length of stay ranged from 1 day to 5 days with a median of 2 days, mean 2.4 days. Patients are discharged with a Foley catheter, which is removed in 7 days to 10 days in the office after a cystogram. Ureteral stents are removed cystoscopically in 6 weeks in the office.
One external iliac vein injury occurred during sharp dissection due to extensive inflammation adhering the ureter to the vein. Pressure was applied on the distal vein with a blunt robotic instrument, and the venotomy was repaired robotically with a 4–0 Monocryl on an RB needle in a figure-of-8 fashion. Anticoagulation was not instituted beyond our standard prophylactic subcutaneous heparin. Another patient had persistent hematuria due to bleeding from the ureterovesical anastomosis, which was controlled with fulguration.
All patients are followed postoperatively with upper-tract imaging, which consists of computed tomography or ultrasound, depending on primary pathology, at 3, 6, 12, 18, and 24 months. Despite normal upper-tract studies, 2 patients complaining of flank pain underwent renal scans with T-1/2 of less than 10 minutes. Ureteral cancers are also followed with regular cystoscopies and urine cytologies.
DISCUSSION
Ureteral surgery requires fine-detail suturing and the ability to change the field of vision from the bladder extending to the kidney. As demonstrated by the patients in this series, this can be accomplished safely using minimally invasive robot-assisted laparoscopic surgery while mimicking the open approach.
The da Vinci robotic tower includes one camera arm and 2 to 3 instrument arms that are controlled remotely by the surgeon sitting at a nonsterile console. Once trained to use the robot-assisted approach, the surgeon can use the da Vinci robot to facilitate the laparoscopic repair. Experienced open surgeons have been shown to have a shorter learning curve with minimally invasive surgery when beginning with the robotic approach first compared with conventional laparoscopy.2–4 Continued use of the robot-assisted technique results in improvement in skill and decreased operating times.3,4
Advantages of the robotic approach include improved dexterity with increased degrees of freedom giving the surgeon the sensation of having wrists rather than lever arms, ease of suturing, enhanced magnification, 3-dimensional visualization from the console providing depth perception similar to that of open surgery, natural hand-eye alignment similar to that of open surgery, increased surgeon comfort with a seat and decreased surgeon hand tremor and/or fatigue compared with these things in traditional operative laparoscopy.1,5–7 Adequate visual field manipulation for these procedures can be accomplished without a change in patient positioning or camera port placement.
We reported the first robot-assisted laparoscopic ureteral reimplantation necessitated by a ureteral injury during radical prostatectomy, which is not included in this current series.8 Other case reports of robot-assisted laparoscopic surgery in adults have included cases of ureteropyelostomy, nephroureterectomy, combined hand-assisted nephrouretectomy and robot-assisted laparoscopic radical prostatectomy, ureteroureterotomy, ureterolysis, ureterocalicostomy, and psoas hitch reimplantation for distal ureteral stenosis.7,9–12 Recently, a series of 12 patients who underwent robot-assisted laparoscopic ureteric reimplantation at multiple institutions was described.13 Another series of robotic reconstruction of the upper urinary tract included 4 patients who underwent ureteral reimplantion.1 Our current series represents the largest single-institution experience of robot-assisted laparoscopic distal ureteral surgery.
Patil et al13 compared their robotic ureteral reimplantation experience with open and laparoscopic series and demonstrated decreased blood loss and length of stay (LOS) with laparoscopy compared with the open approach. Despite the dissimilar indications for surgery and the inclusion of pelvic node dissection in many of our cases, our results are similar to this series with regards to total operating-room times (208 minutes vs.189 minutes), robot times (173 minutes vs. 157 minutes), LOS (4.3 days vs. 2.4 days), and EBL (48 cc vs 82 cc).
In appropriately selected patients, distal ureterectomy is an acceptable modality for distal ureteral tumors. Our series contains 5 patients who underwent robotic distal ureterectomy for transitional cell cancer, making this the largest series to date of robotic distal ureterectomies for cancer. There were 2 ipsilateral recurrences in the renal pelvis thereafter treated with hand-assisted laparoscopic nephroureterectomy. All patients are alive and free of disease. These results compare favorably with results of a laparoscopic series by Roupret et al,14 in which 6 patients underwent laparoscopic distal ureterectomy with similar operating room times, recurrence rates, and patent anastomosis.
Prior to the advent of robotic surgery, conventional laparoscopy had also been utilized in this area. Published reports14–20 have included a number of surgical procedures on the ureter, including segmental or distal ureterectomy with reanastomosis, ureteroureterostomy, ileal ureter, Boari flap, and ureteroneocystotomy with or without vesicopsoas hitch.
Both routes of minimally invasive surgery offer advantages compared with open surgery, including improved magnification, lower blood loss, less pain, and visualization and cosmesis.17,18,21 Additional benefits may include shorter hospitalization and faster return to work, although these have not yet been adequately studied. Research comparing the 2 minimally invasive techniques is difficult considering the low number of patients available for such studies, particularly for ureteral surgery. In a study reviewing 29 pediatric patients undergoing pyeloplasty from a single surgeon, anastomoses sewn during robot-assisted surgery were no different than ones sewn using conventional laparoscopic techniques.5 Another study21 suggests that patients undergoing robot-assisted prostate surgery have better outcomes than patients who have conventional laparoscopy, although this is difficult to generalize to other procedures.
Limitations to robotic surgery may include the absence of tensile feedback, the learning curve, and time investment associated with new surgical technology, need for an experienced bedside assistant, and the cost and training involved in launching a robotic surgery program.1,5 Some of these drawbacks may be resolved with future technology.
CONCLUSION
Robot-assisted laparoscopic surgery offers advantages for ureteral surgeries and should be considered as an option.
Contributor Information
Megan O. Schimpf, Division of Urogynecology, Department of Obstetrics & Gynecology, Hartford Hospital, Hartford, Connecticut, USA..
Joseph R. Wagner, Department of Urology, Connecticut Surgical Group, Hartford Hospital, Hartford, Connecticut, USA..
References:
- 1.Mufarrij PW, Shah OD, Berger AD, Stifelman MD. Robotic reconstruction of the upper urinary tract. J Urol. 2007;178:2002–2005 [DOI] [PubMed] [Google Scholar]
- 2.Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: Initial experience with laparoscopic radical prostatectomy. J Urol. 2003;170:1738–1741 [DOI] [PubMed] [Google Scholar]
- 3.Yohannes P, Rotariu P, Pinto P, Smith AD, Lee BR. Comparison of robotic versus laparoscopic skills: Is there a difference in the learning curve? Urology. 2002;60:39–45 [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Satava RM, Pellegrini CA, Sinanan MN. Robotic surgery: Identifying the learning curve through objective measurement of skill. Surg Endosc. 2003;17:1744–1748 [DOI] [PubMed] [Google Scholar]
- 5.Franco I, Dyer LL, Zelkovic P. Laparoscopic pyeloplasty in the pediatric patient: Hand-sewn anastomosis versus robotic-assisted anastomosis – Is there a difference? J Urol. 2007;178:1483–1486 [DOI] [PubMed] [Google Scholar]
- 6.Melamud O, Eichel L, Turbow B, Shanberg A. Laparoscopic vesicovaginal fistula repair with robotic reconstruction. Urology. 2005;65:163–166 [DOI] [PubMed] [Google Scholar]
- 7.De Naeyer G, Van Migem P, Schatteman P, Carpentier P, Fonteyne E, Mottrie AM. Pure Robot-assisted psoas hitch ureteral reimplantation for distal-ureteral stenosis. J Endourol. 2007;21:618–620 [DOI] [PubMed] [Google Scholar]
- 8.Dinlenc CZ, Gerber E, Wagner JW. Ureteral reimplantation during robot-assisted laparoscopic radical prostatectomy. J Urol. 2004;172:905. [DOI] [PubMed] [Google Scholar]
- 9.Duchene DA, Theil DD, Winfield HW. Robotic-assisted laparoscopic ureteropyelostomy for treatment of prostatitis secondary to ectopic ureteral insertion of a completely duplicated collecting system. J Endourol. 2007;21:455–457 [DOI] [PubMed] [Google Scholar]
- 10.Uberoi J, Harnisch B, Sethi AS, Babayan RK, Wang DS. Robot-assisted laparoscopic distal ureterectomy and ureteral reimplantation with psoas hitch. J Endourol. 2007;21(4):368–373 [DOI] [PubMed] [Google Scholar]
- 11.Finley DS, Melamud O, Ornstein DK. Combined robot-assisted laparoscopic nephroureterectomy and radical prostatectomy. J Endourol. 2007;21:411–414 [DOI] [PubMed] [Google Scholar]
- 12.Nanigian DK, Smith W, Ellison LM. Robot-assisted laparoscopic nephroureterectomy. J Endourol. 2006;20:463–466 [DOI] [PubMed] [Google Scholar]
- 13.Patil NN, Mottrie A, Sundaram B, Patel VR. Robotic-assisted laparoscopic ureteral reimplantation with psoas hitch: a multi-institutional, multinational evaluation. Urology. 2008;72(1):47–50 [DOI] [PubMed] [Google Scholar]
- 14.Rouprêt M, Harmon JD, Sanderson KM, et al. Laparoscopic distal ureterectomy and anastomosis for management of low-risk upper urinary tract transitional cell carcinoma: preliminary results. BJU Inter. 2007;99:623–627 [DOI] [PubMed] [Google Scholar]
- 15.Gerber E, Dinlenc CZ, Wagner JR. Laparoscopic distal ureterectomy for low-grade transitional cell carcinoma. J Urol. 2003;169:2295. [DOI] [PubMed] [Google Scholar]
- 16.Gill IS, Savage SJ, Senagore AJ, Tak Sung G. Laparoscopic ileal ureter. J Urol. 2000;163:1199–1202 [PubMed] [Google Scholar]
- 17.Nezhat CH, Malik S, Nezhat F, Nezhat C. Laparoscopic ureteroneocystotomy and vesicopsoas hitch for infiltrative endometriosis. JSLS. 2004;8:3–7 [PMC free article] [PubMed] [Google Scholar]
- 18.Modi P, Goel R, Dodiya S. Laparoscopic ureteroneocystotomy for distal ureteral injuries. Urology. 2005;66:751–753 [DOI] [PubMed] [Google Scholar]
- 19.Fugita OE, Dinlenc C, Kavoussi L. The laparoscopic Boari flap. J Urol. 2001;166:51–53 [PubMed] [Google Scholar]
- 20.Chung H, Jeong BC, Kim HH. Laparoscopic ureteroneocystotomy with vesicopsoas hitch: Nonrefluxing ureteral reimplantation using cystoscopy-assisted submucosal tunneling. J Endourol. 2006;20:632–638 [DOI] [PubMed] [Google Scholar]
- 21.Menon M, Shrivastava A, Tewari A. Laparoscopic radical prostatectomy: Conventional and robotic. Urology. 66(Suppl 5A):101–104, 2005 [DOI] [PubMed] [Google Scholar]