Abstract
Background and Objectives:
Pseudomyxoma peritonei results from ovarian and appendiceal mucinous tumors. Cyst rupture results in intraabdominal mucin accumulation, leading to abdominal distension. No effective treatment has yet been established. Pseudomyxoma peritonei is generally associated with a poor prognosis. In a recent Mayo Clinic report, the 5-year survival rate for this disease was 53% and the 10-year survival rate was 32%, while the Memorial Sloan-Kettering Cancer Center reported 5- and 10-year survival rates of 75% and 10%.
Methods and Results:
In this report, we describe 4 patients with a laparoscopically confirmed recurrence of pseudomyxoma peritonei who subsequently underwent repeated laparoscopic mucin removal.
Conclusion:
Because laparoscopic surgery can be performed frequently, it appears that laparoscopic surgery, a minimally invasive procedure, greatly improves the quality of life of patients with pseudomyxoma peritonei.
Keywords: Pseudomyxoma peritonei, Laparoscopic surgery, Mucin removal, Quality of life
INTRODUCTION
Pseudomyxoma peritonei, a mucinous cancer primarily of ovarian origin, was first described by Rokitansky in 1892, although the term “pseudomyxoma peritonei” was first used by Werth.1,2 In 1901, Frankel reported a similar pathology in association with a cyst of the appendix.3 Since then, the term has been used for various mucusproducing tumors characterized by abundant extracellular mucin. This disease occurs most commonly in females 40 to 60 years of age and originates from mucinous tumors of the ovary or appendix. It is a rare disease characterized by abdominal swelling caused by the mucinous material collected in the entire abdominal cavity following cyst rupture, whether benign or malignant. In many cases, examination reveals an elevated rate of erythrocyte sedimentation and elevated levels of carcinoembryonic antigen. Diagnostic findings of fluid collection and tumor images are often obtained through advanced imaging technologies including supersonography or CT.4 Recently, MRI has also been reported as useful.5 However, the definitive diagnosis is made by the confirmation of intraperitoneal gelatinous mucus. Surgical resection of the primary lesion is the conventional treatment.
Recurrences may be observed, and when they are, complete removal of mucus-producing cells disseminated in the peritoneal cavity is difficult, and multiple abdominal surgeries are often required. In some cases, prophylactic removal of pelvic viscera is performed. The prognosis is generally poor, because many cases, whether benign or malignant initially, recur repeatedly regardless of further treatment.
Herein, we report on 4 patients diagnosed as having pseudomyxoma peritonei who underwent repeated mucus removal via laparoscopy.
CASE REPORT ONE
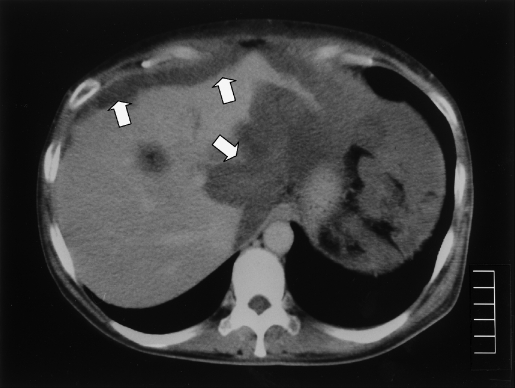
This patient was a 47-year-old, G0P0 (gravidity = 0, parity = 0) female. She underwent a partial appendectomy for treatment of appendicitis when she was 7 years old. At that time, a portion of the appendix was not resected because of intense inflammation. At age 23, she underwent abdominal surgery in our department to remove a right ovarian tumor. The tumor was 3 cm × 3 cm in diameter with a calcified shell and was determined to be pseudomyxoma peritonei of primary appendiceal origin. The patient was treated with oral administration of cyclophosphamide for 2 years and was followed by ultrasonography every other year on an outpatient basis. At age 33, an abnormal shadow in the Douglas cavum was found on ultrasonography and CT. The first laparoscopic surgery was performed, and recurrence of pseudomyxoma peritonei localized in the uterus and uterine appendages was confirmed. Mucus was removed as much as possible under laparoscopy. No abnormality was observed at the site of the previous tumor removal. Again, surgery was followed by oral cyclophosphamide administration for one year. One year later, at age 34, a second laparoscopic surgery was performed with the intention of confirming the rate at which the lesion was expanding. The lesions in the uterus and appendages remained unchanged, but additional lesions were observed around the liver. Again, mucus was maximally removed. Then, the patient was placed under follow-up on an outpatient basis and underwent ultrasound imaging every other year. Subsequently, at age 43, she experienced intensified abdominal distention and anorexia. CT examination revealed an abnormal shadow (Figure 1). A third laparoscopic surgery was performed, and expansions of lesions were observed throughout the peritoneal cavity with formation of masses on the serous surface of the sigmoid and gastric corpus. The peritoneal cavity was washed with low-molecular-weight dextran, and mucus was removed to the maximum extent possible. During the next 2 years, the patient experienced abdominal distention, and fourth and fifth laparoscopic surgeries were performed when she was 44 and 45 years old, respectively. On each occasion, mucus had accumulated throughout the peritoneal cavity, and 9.6 L and 5.6 L of mucus was removed with saline at the fourth and fifth surgeries, respectively.
Figure 1.
Computed Tomographic scan of accumulated mucus (Case 1).
The patient has undergone a total of 5 laparoscopic surgeries to date and has survived 25 years since initially presenting with pseudomyxoma peritonei.
CASE REPORT TWO
A 56-year-old, G2P2 female had been seen in the department of internal medicine at our hospital when she was 51years of age. Her chief complaint of abdominal distention was thought to result from pseudomyxoma peritonei, diagnosed by CT (Figure 2). Laparoscopic surgery was performed for intraperitoneal observation and removal of mucus. A mass near the appendix and mucus spreading throughout the peritoneal cavity were confirmed, and the mucus was removed. The second and third laparoscopic surgeries were performed when the patient was 52 and 53 years old, respectively, because of continued complaints of abdominal distention. On each occasion the accumulated mucus was removed to the maximum extent possible, and 6.8L and 6.7L of mucosa was removed at the second and third surgery, respectively. At present, this patient is being followed up on an outpatient basis.
Figure 2.
Computed Tomographic image of accumulated mucus (Case 2).
CASE REPORT THREE
A 75-year-old, 2G2P, female, at age 71 was referred to our department from another hospital where an accumulation of ascites and the presence of masses in the left lower abdominal quadrant had been observed. CT examination revealed pseudomyxoma peritonei, and laparoscopic surgery was performed. Mucus had spread throughout the peritoneal cavity, and the appendiceal capsule had ruptured. Laparoscopic appendicectomy was performed, and mucus that had accumulated up to the volume of 0.5L was removed as far as possible. Two years later, at the age of 74, the patient developed abdominal distention, and CT confirmed the presence of pseudomyxoma peritonei spreading to the upper abdomen. The second laparoscopic surgery was performed. This patient is currently being followed up on an outpatient basis.
CASE REPORT FOUR
A 61-year-old, 2G2P female, at age 60 visited her local hospital with a chief complaint of abdominal distention and received a diagnosis of inoperable pancreatic cancer. She sought a second opinion and received a CT diagnosis of suspected pseudomyxoma peritonei. She was referred to our hospital where laparoscopic surgery revealed masses throughout the peritoneal cavity and abundant accumulation of mucus at the volume of 10.8 L. Mucus was removed to the maximum extent possible. Six months later, the second laparoscopic surgery was performed because of the recurrence of abdominal distention and weight gain of 18 kg from after the previous surgery. Mucus accumulated to the volume of 16.5 L was removed as much as possible. It was noted that the size of the previously observed abdominal masses had increased. Six months later, the third laparoscopic surgery was performed because of increased abdominal distention and weight gain of 20 kg from the previous time. Mucus accumulated to the volume of 16 L was removed as much as possible. Recently, the rate of mucus accumulation has increased.
DISCUSSION
Pseudomyxoma peritonei is generally considered a disease with a poor prognosis, with 5-year and 10-year survival rates of 53% and 32%, respectively, according to a recent report from the Mayo Clinic,6 and 75% and 10%, respectively, according to a report from the Memorial Sloan Kettering Cancer Center.7
Sugarbaker et al8 reported that the treatment of pseudo-myxoma peritonei is principally based on resection of the primary lesion and removal of mucus by laparotomy. Because complete removal of mucus-producing cells from the peritoneal cavity is rarely achieved, laparotomy must be repeated at intervals determined by the rate of mucus accumulation. In addition, a recurrence rate of over 90% is reported even after the removal.9 Consequently, patients with this disease, which has a poor prognosis with repeated recurrence, must undergo multiple highly invasive procedures.
Nonsurgical treatment approaches have been reported recently, including systemic and intraperitoneal applications of chemical therapy using 5-flurouracil (5FU) or cisplatin (CDDP).6,10 Various therapies including continuous hyperthermic peritoneal perfusion, in which the peritoneal cavity is perfused with heated saline solution, and intraperitoneal hyperthermic chemotherapy, in which anti-cancer agents are included with the heated saline perfusion have also been attempted.7,11–14 However, none of them can greatly improve the prognosis.
The major symptom of pseudomyxoma peritonei is abdominal distention resulting from accumulation of mucus. To alleviate this symptom, mucus removal is performed. However, the mucus is often highly viscous, and its removal by abdominal puncture is difficult. There are various reports on mucolytic therapy. Green et al15 described a mucolytic therapy using a 5% glucose solution to dissolve mucin-like mucus. Others16 have reported that the mucolytic effect of such solutions was not superior to that of saline or low-molecular-weight dextran. In some of the cases presented here, in which mucus removal through the suction tube was difficult, we used low-molecular-weight dextran or saline.
Usually, intraperitoneal mucus cannot be completely removed, and the prognosis of this disease remains poor. At present, frequent surgical laparotomy is performed. In this situation, laparoscopic procedures have been reported for observing the peritoneal cavity.17–19 Among them, mucus removal by trocar insertion and postoperative administration of an anti-cancer agent via that portal site have also been reported.18,19
Laparoscopy, which provides a view of the entire peritoneal cavity, is also useful for observing the progression of the disease, which may recur repeatedly in the entire abdominal cavity. The first trocar is inserted at the umbilicus to locate the mucus pool, and the second trocar is inserted for aspiration and cleaning. The trocar can be inserted selectively from sites including the right and left sides of the pubic region and the upper and lower abdomen along the median line, depending on the mucus-pooling site, to remove the mucus from the entire peritoneal cavity. In laparotomy, on the contrary, a large incision is needed to view the entire cavity; therefore, the procedure is more invasive for the patient. Consequently, the quality of life of patients with pseudomyxoma peritonei would be improved by repeatedly performing minimally invasive laparoscopic surgery.
CONCLUSION
In this article, we report on 4 patients who underwent repeated laparoscopic mucus removal. Based on this experience, we recommend laparoscopic surgery as a means to alleviate the symptoms of abdominal distention. We plan to continue performing intraperitoneal observation and mucus removal by laparoscopy in these cases.
References:
- 1.Weaver CH. Mucocele of appendix with pseudomucinous degeneration. Am J Surg. 1937;36:523–526 [Google Scholar]
- 2.Werth R. Klinische und Anatomische Untersuchungen zur Lehre den Bauchgeshwullsten und der Laparotomie. Arch Gynecol Obstet. 1884;24:100–118 [Google Scholar]
- 3.Frankel E. Uber das sogenannte pseudomyxoma peritonei. Med Wochenschr. 1901;48:965–970 [Google Scholar]
- 4.Yeh HC, Shafir MK, Slater G, Meyer RJ, Cohen BA, Geller SA. Ultrasonography and computed tomography in pseudomyxoma peritonei. Radiology. 1984;153(2):507–510 [DOI] [PubMed] [Google Scholar]
- 5.Buy JN, Malbec L, Ghossain MA, Guinet C, Ecoiffier J. Magnetic resonance imaging of pseudomyxoma peritonei. Eur J Radiol. 1989;9(2):115–118 [PubMed] [Google Scholar]
- 6.Gough DB, Donohue JH, Schutt AJ, et al. Pseudomyxoma peritonei: long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JW, Kemeny N, Caldwell C, Banner P, Sigurdson E, Huvos A. Pseudomyxoma peritonei of appendiceal origin. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1992;70(2):396–401 [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker PH, Ronnett BM, Archer A, et al. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233–280 [PubMed] [Google Scholar]
- 9.Miner TJ, Shia J, Jaques DP, Klimstra D, Brennan NF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei. An analysis of surgical therapy. Ann Surg. 2005;241:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterworth SA, Panton ON, Klaassen DJ, Shah AM, McGregor GI. Morbidity and mortality associated with intraperitoneal chemotherapy for Pseudomyxoma peritonei. Am J Surg. 2002;183(5):529–532 [DOI] [PubMed] [Google Scholar]
- 11.Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–260 [PubMed] [Google Scholar]
- 12.Benoit L, Cheynel N, Ortega-Deballon P, Giacoma GD, Chauffert B, Rat P. Closed hyperthermic intraperitoneal chemo-therapy with open abdomen: a novel technique to reduce exposure of the surgical team to chemotherapy drugs. Ann Surg Oncol. 2008;15(2):542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran BJ, Meade B, Murphy E. Hyperthermic intraperitoneal chemotherapy and cytoreductive surgery for peritoneal carcinomatosis of colorectal origin: a novel treatment strategy with promising results in selected patients. Colorectal Dis. 2006;8(7):544–550 [DOI] [PubMed] [Google Scholar]
- 14.Nasr MF, Kemp GM, Given FT., Jr Pseudomyxoma peritonei: treatment with intraperitoneal 5-flurouracil. Eur J Gynaecol Oncol. 1993;14(3):213–217 [PubMed] [Google Scholar]
- 15.Green N, Gancedo H, Smith R, Bernett G. Pseudomyxoma peritonei-nonoperative management and biochemical findings. A case report. Cancer. 1975;36(5):1834–1837 [DOI] [PubMed] [Google Scholar]
- 16.Shyr YM, Su CH, Wang HC, Lo SS, Lui WY. Pseudomyxoma peritonei: does a true mucolytic agent exist? In vitro and in vivo studies. Am Surg. 1995;61:265–270 [PubMed] [Google Scholar]
- 17.Georgescu S, Angheluţ A, Andronic D, et al. Mucinous digestive tumors. Case reports and review of the literature. Rom J Gastroenterol. 2002;11(3):213–218 [PubMed] [Google Scholar]
- 18.Raj J, Urban LM, ReMine SG, Raj PK. Laparoscopic management of pseudomyxoma peritonei secondary to adenocarcinoma of the appendix. J Laparoendosc Adv Surg Tech A. 1999;9(3):299–303 [DOI] [PubMed] [Google Scholar]
- 19.Chui CJ. pseudomyxoma peritonei diagnosed by peritoneoscopy. Aust N Z J Surg. 1975;45(3):277–279 [DOI] [PubMed] [Google Scholar]