Abstract
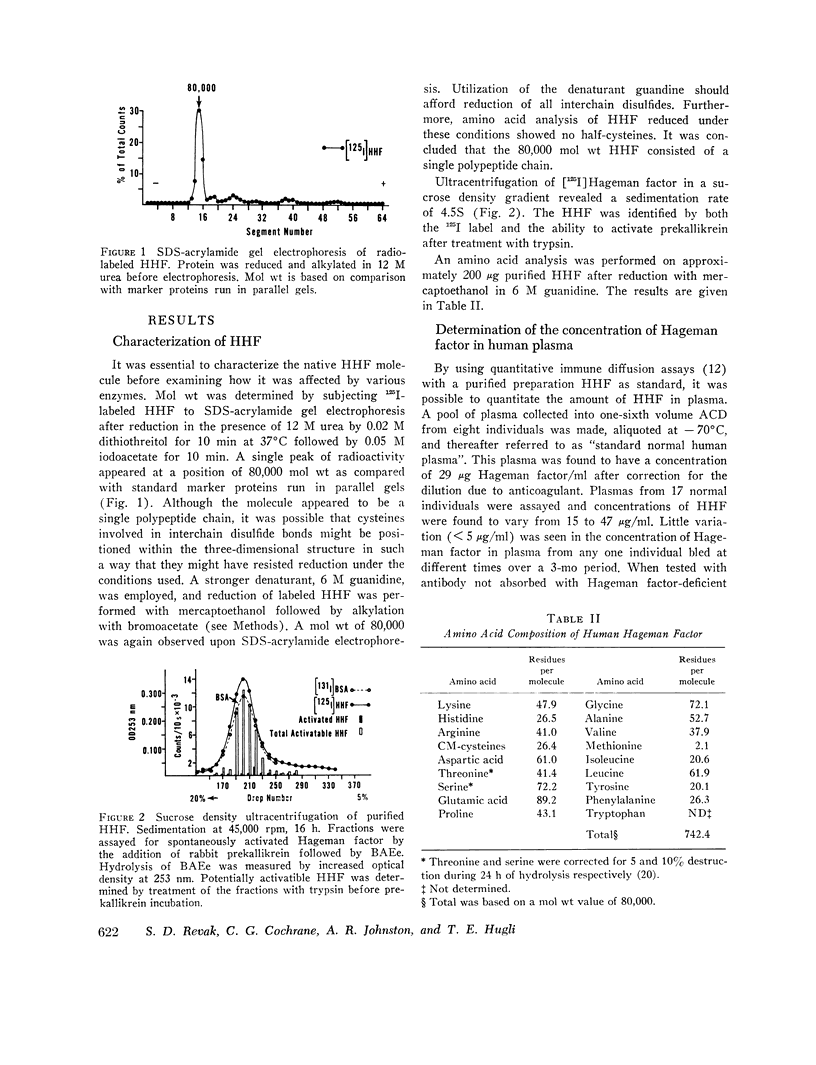
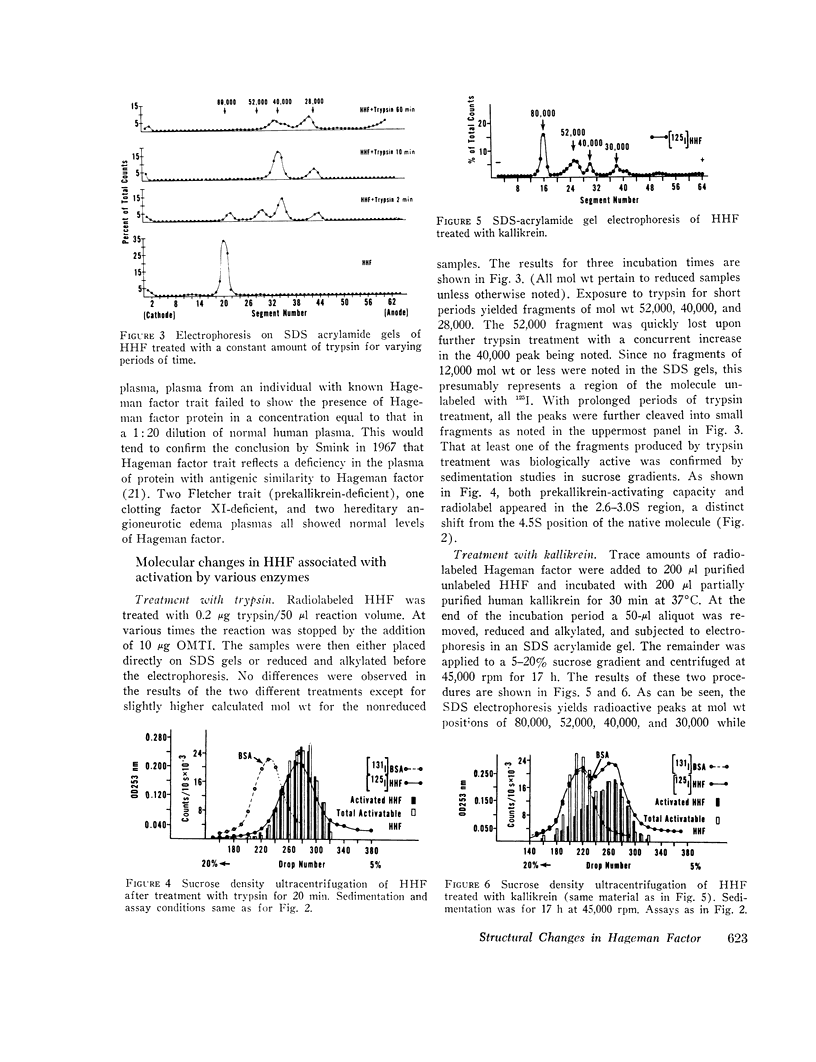
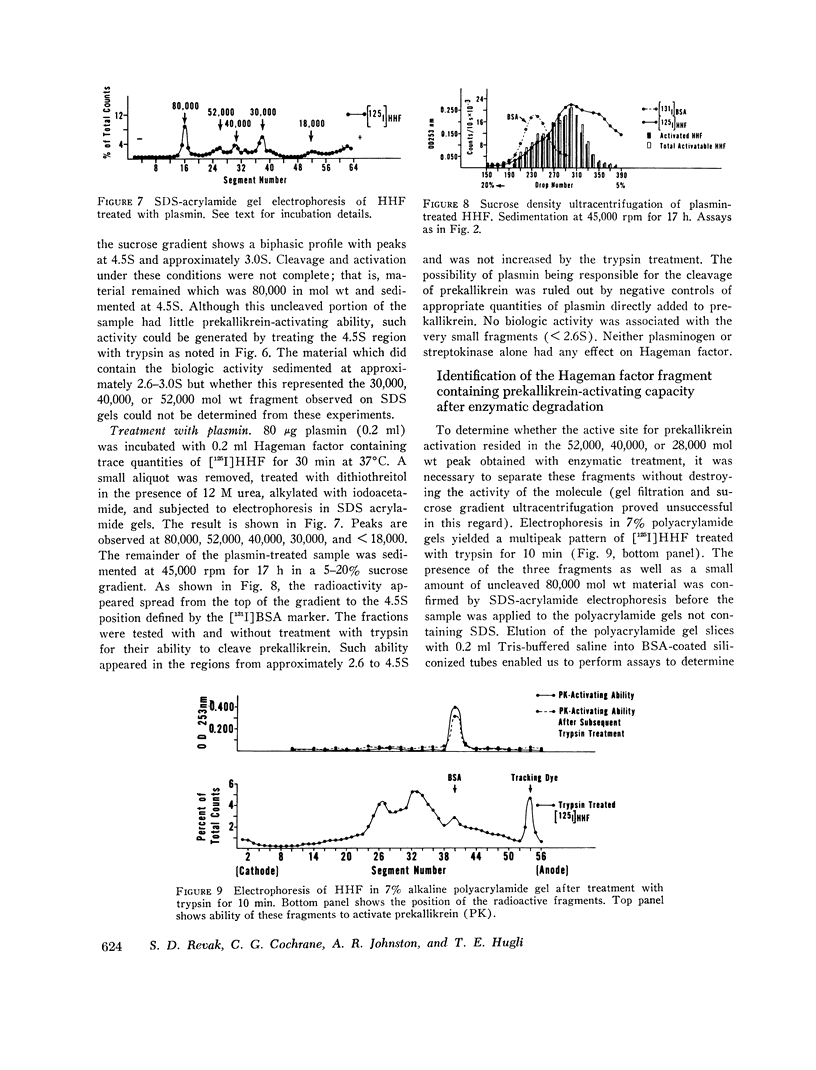
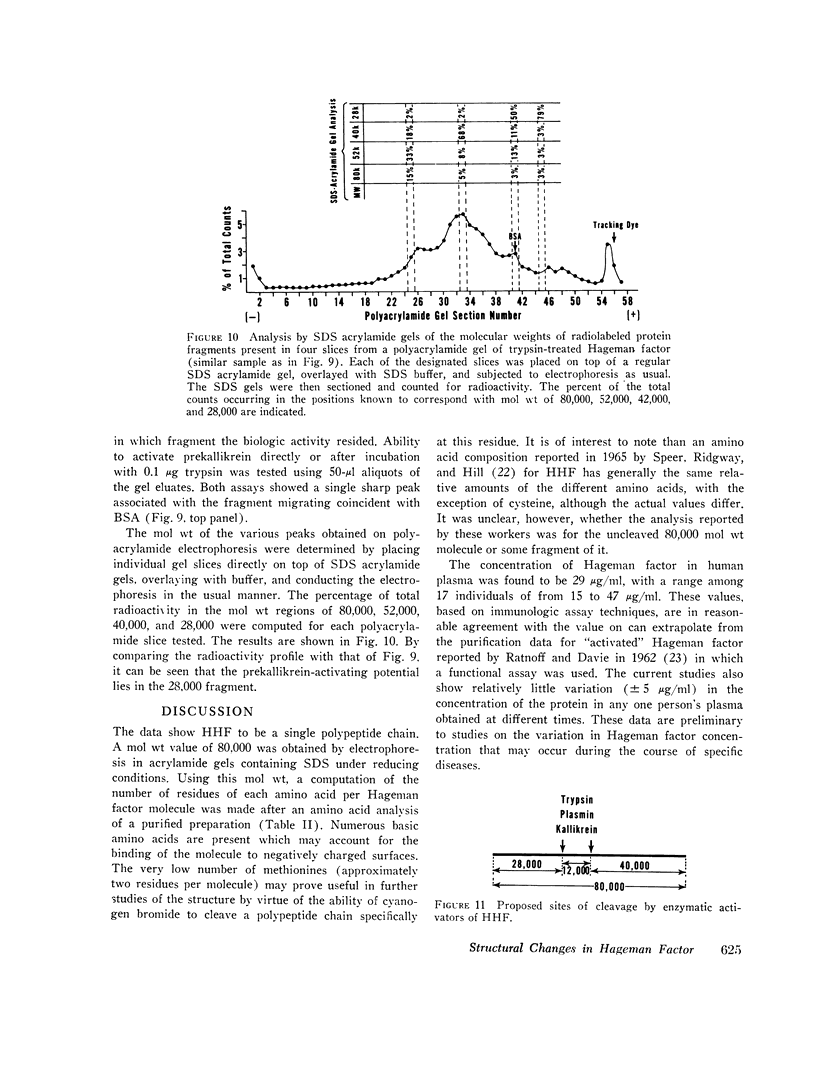
The structure of Hageman factor, isolated from human plasma, was analyzed before and after enzymatic activation. The purified molecule is a single polypeptide chain of 80,000 molecular weight (mol wt) sedimenting at 4.5S. An amino acid analysis has been performed. The concentration of Hageman factor in normal human plasma was found to be 29 μg/ml with variation between individuals ranging from 15 to 47 μg/ml. Treatment of the molecule with kallikrein, plasmin, or trypsin resulted in cleavage at two primary sites, yielding fragments of 52,000, 40,000, and 28,000 mol wt. No further changes occurred in the fragments with subsequent reduction. Prekallikrein-activating ability was associated exclusively with the 28,000 moiety.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrowes C. E., Movat H. Z., Soltay M. J. The kinin system of human plasma. VI. The action of plasmin. Proc Soc Exp Biol Med. 1971 Dec;138(3):959–966. doi: 10.3181/00379727-138-36027. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Wuepper K. D. The first component of the kinin-forming system in human and rabbit plasma. Its relationship to clotting factor XII (Hageman Factor). J Exp Med. 1971 Oct 1;134(4):986–1004. doi: 10.1084/jem.134.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- IATRIDIS S. G., FERGUSON J. H. Actie Hageman factor: a plasma lysokinase of the human fibrinolytic system. J Clin Invest. 1962 Jun;41:1277–1287. doi: 10.1172/JCI104590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. J., Kline D. L., Alkjaersig N. Assay methods and standard preparations for plasmin, plasminogen and urokinase in purified systems, 1967-1968. Thromb Diath Haemorrh. 1969 Apr 30;21(2):259–272. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A pre-albumin activator of prekallikrein. J Immunol. 1970 Oct;105(4):802–811. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. The fibrinolytic pathway of human plasma. Isolation and characterization of the plasminogen proactivator. J Exp Med. 1972 Dec 1;136(6):1378–1393. doi: 10.1084/jem.136.6.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS J. Activation of plasma by contact with glass: evidence for a common reaction which releases plasma kinin and initiates coagulation. J Physiol. 1958 Nov 10;144(1):1–22. doi: 10.1113/jphysiol.1958.sp006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Ogston D., Ogston C. M., Ratnoff O. D., Forbes C. D. Studies on a complex mechanism for the activation of plasminogen by kaolin and by chloroform: the participation of Hageman factor and additional cofactors. J Clin Invest. 1969 Oct;48(10):1786–1801. doi: 10.1172/JCI106145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W., MALLETT D. L. Studies on the action of Hageman factor: evidence that activated Hageman factor in turn activates plasma thromboplastin antecedent. J Clin Invest. 1961 May;40:803–819. doi: 10.1172/JCI104314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W. The purification of activated Hageman factor (activated factor XII). Biochemistry. 1962 Nov;1:967–975. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., ROSENBLUM J. M. Role of Hageman factor in the initiation of clotting by glass; evidence that glass frees Hageman factor from inhibition. Am J Med. 1958 Aug;25(2):160–168. doi: 10.1016/0002-9343(58)90023-8. [DOI] [PubMed] [Google Scholar]
- Smink M. M., Daniel T. M., Ratnoff O. D., Stavitsky A. B. Immunologic demonstration of a deficiency of Hageman factor-like material in Hageman trait. J Lab Clin Med. 1967 May;69(5):819–832. [PubMed] [Google Scholar]
- Speer R. J., Ridgway H., Hill J. M. Activated human Hageman factor (XII). Thromb Diath Haemorrh. 1965 Sep 1;14(1-2):1–11. [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER M. E., ATNOFF O. D. Role of Hageman factor in the activation of vasodilator activity in human plasma. Nature. 1961 Oct 14;192:180–181. doi: 10.1038/192180a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wuepper K. D., Cochrane C. G. Plasma prekallikrein: isolation, characterization, and mechanism of activation. J Exp Med. 1972 Jan;135(1):1–20. doi: 10.1084/jem.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


