Abstract
Cell suspensions enriched in human blood monocytes, obtained from normal peripheral blood by sedimentation on sodium diatrizoate-Ficoll gradients or from the blood of patients with neutropenia and monocytosis, accumulated malonyldialdehyde, a labile catabolite of lipid peroxidation, during incubations with polystyrene beads or heat-killed Staphylococcus epidermidis. Mixed blood leukocytes principally composed of granulocytes or granulocytes purified by density gradient sedimentation did not accumulate malonyldialdehyde during incubations with these particles, but did when ingesting particles containing linolenate. The phospholipid fatty acid composition of monocyte-enriched and purified granulocyte preparations from the same donors were compared. The molar fraction of arachidonate (20:4) in phospholipids from monocyte-rich preparations was 62% greater than that of purified granulocytes. The findings indicate that human monocytes, possibly because of a greater content of polyunsaturated fatty acids in their membranes, peroxidize a greater quantity of endogenous lipids than granulocytes during endocytosis. Normal human granulocytes have the capacity to peroxidize ingested lipids. However, mixed leukocytes from two patients with chronic granulomatous disease produced little malonyldialdehyde when engulfing linolenate-containing particles. Therefore the capacity to peroxidize lipid is related to cellular oxygen metabolism, a function in which chronic granulomatous disease granulocytes are dificient.
Malonyldialdehyde chemically prepared by hydrolysis of tetramethoxypropane, by extraction from peroxidized linolenic acid, or purified from extracts of phagocytizing rabbit alveolar macrophages had bactericidal activity against Escherichia coli and S. epidermidis. Therefore, toxic catabolites of lipid hydroperoxides may potentiate the bactericidal activity of hydrogen peroxide in mononuclear phagocytes.
Full text
PDF


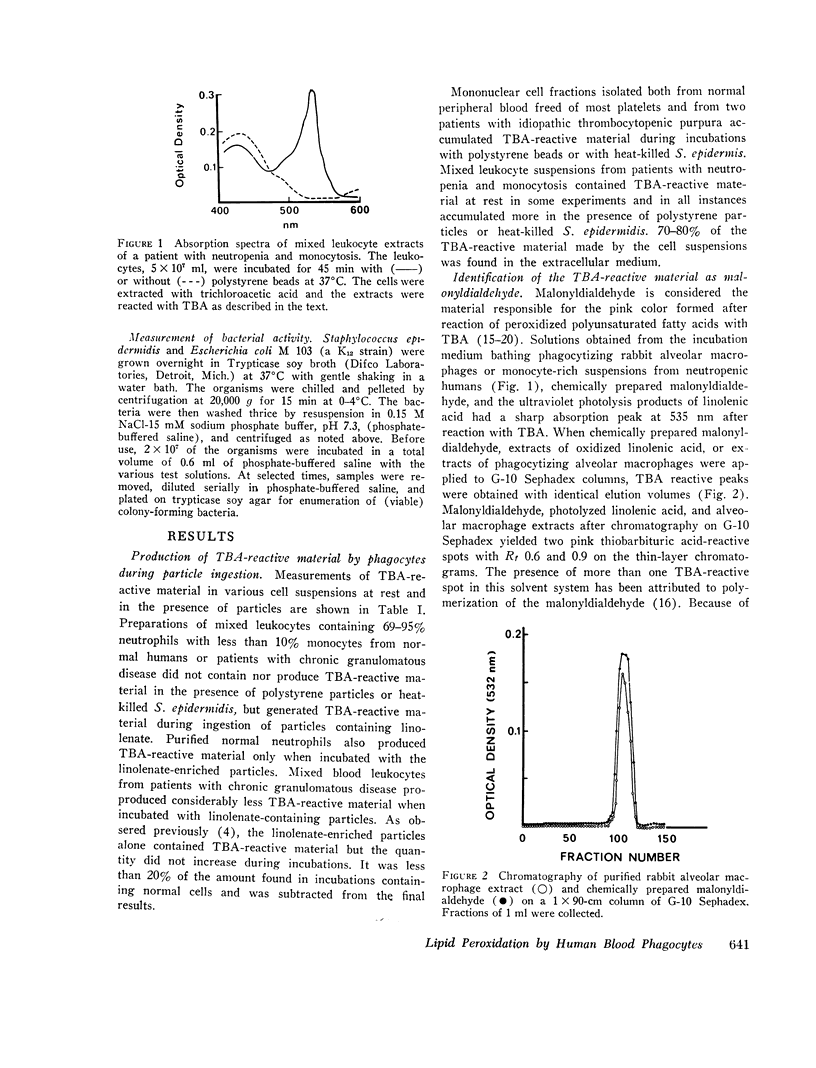
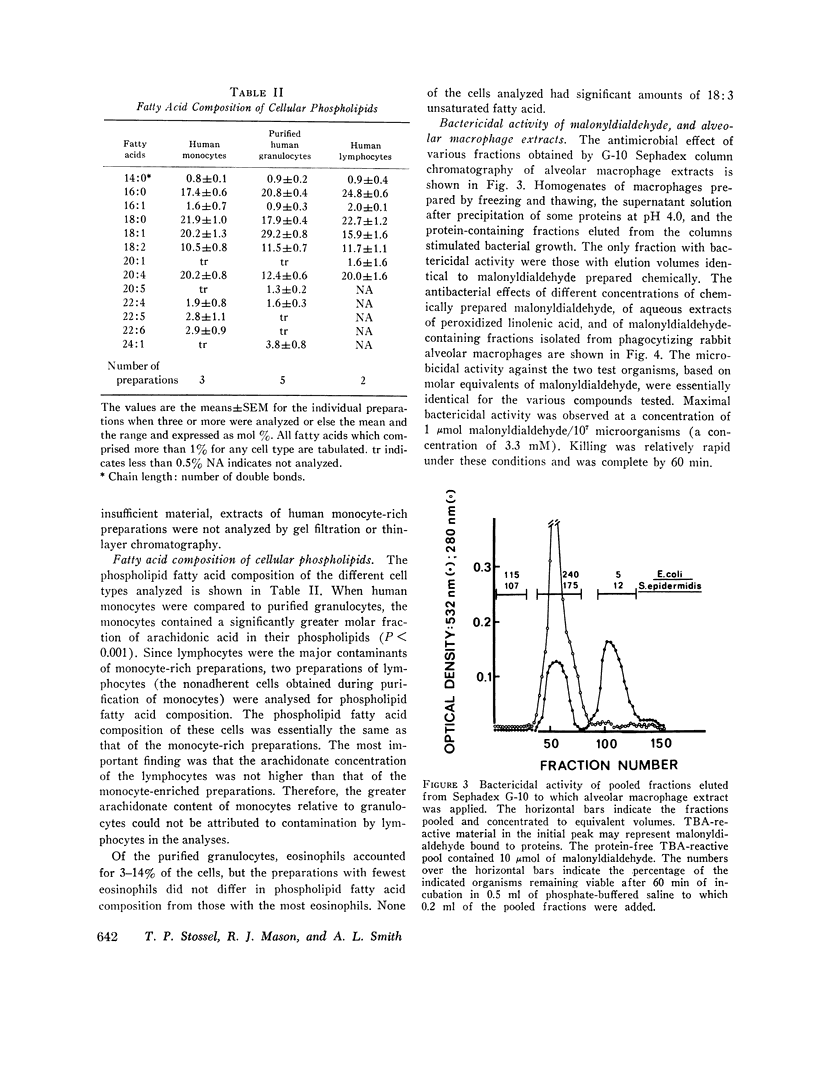
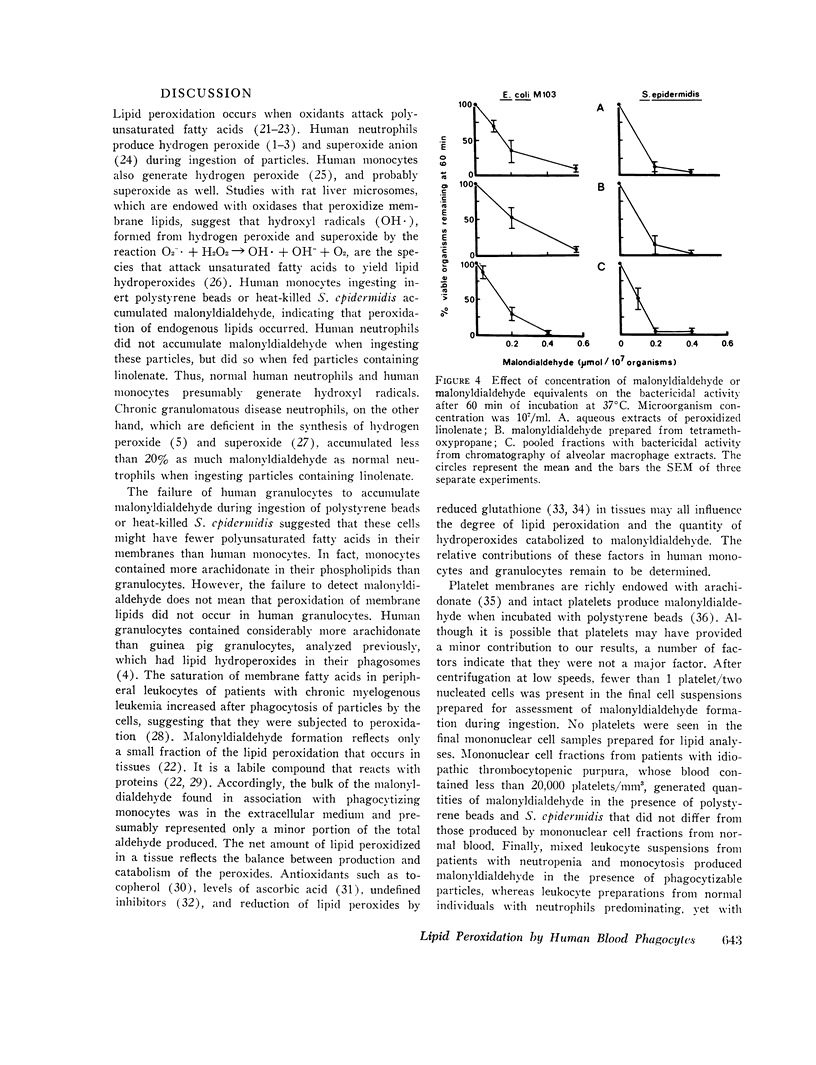


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER A. A. Inhibition of lipid peroxide formation by vertebrate blood serum. Arch Biochem Biophys. 1961 Jan;92:38–43. doi: 10.1016/0003-9861(61)90215-6. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Johnston R. B., Jr Monocyte function in children with neutropenia and chronic infections. Blood. 1972 Jul;40(1):31–41. [PubMed] [Google Scholar]
- Barber A. A., Bernheim F. Lipid peroxidation: its measurement, occurrence, and significance in animal tissues. Adv Gerontol Res. 1967;2:355–403. [PubMed] [Google Scholar]
- Brooks B. R., Klamerth O. L. Interaction of DNA with bifunctional aldehydes. Eur J Biochem. 1968 Jul;5(2):178–182. doi: 10.1111/j.1432-1033.1968.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Christophersen B. O. The inhibitory effect of reduced glutathione on the lipid peroxidation of the microsomal fraction and mitochondria. Biochem J. 1968 Jan;106(2):515–522. doi: 10.1042/bj1060515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Grinna L. S., Barber A. A. Lipid peroxidation in livers and kidneys from young and old rats. Biochem Biophys Res Commun. 1973 Dec 10;55(3):773–779. doi: 10.1016/0006-291x(73)91211-4. [DOI] [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- Harris R. S., Bunker J. W., Milas N. A. The Germicidal Activity of Vapors from Irradiated Oils. J Bacteriol. 1932 Jun;23(6):429–435. doi: 10.1128/jb.23.6.429-435.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L. The metabolism of leukocytes. Semin Hematol. 1968 Apr;5(2):156–165. [PubMed] [Google Scholar]
- Khandwala A., Gee J. B. Linoleic acid hydroperoxide: impaired bacterial uptake by alveolar macrophages, a mechanism of oxidant lung injury. Science. 1973 Dec 28;182(4119):1364–1365. doi: 10.1126/science.182.4119.1364. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Intraleukocytic microbicidal defects. Annu Rev Med. 1971;22:39–62. doi: 10.1146/annurev.me.22.020171.000351. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE R. D., SCHOENBERG M. D. THE RESPONSE OF THE HISTIOCYTES AND MACROPHAGES IN THE LUNGS OF RABBITS INJECTED WITH FREUND'S ADJUVANT. Br J Exp Pathol. 1964 Oct;45:488–497. [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Marcus A. J., Ullman H. L., Safier L. B. Lipid composition of subcellular particles of human blood platelets. J Lipid Res. 1969 Jan;10(1):108–114. [PubMed] [Google Scholar]
- Mason R. J., Stossel T. P., Vaughan M. Lipids of alveolar macrophages, polymorphonuclear leukocytes, and their phagocytic vesicles. J Clin Invest. 1972 Sep;51(9):2399–2407. doi: 10.1172/JCI107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- McCay P. B., Poyer J. L., Pfeifer P. M., May H. E., Gilliam J. M. A function for -tocopherol: stabilization of the microsomal membrane from radical attack during TPNH-dependent oxidations. Lipids. 1971;6(5):297–306. [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma M., Steiner M., Baldini G. Studies on lipid peroxides in platelets. II. Effect of aggregating agents and platelet antibody. J Lab Clin Med. 1971 May;77(5):728–742. [PubMed] [Google Scholar]
- Pfeifer P. M., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. V. Use of erythrocytes to demonstrate enzyme-dependent production of a component with the properties of a free radical. J Biol Chem. 1971 Nov;246(21):6401–6408. [PubMed] [Google Scholar]
- Reiss U., Tappel A. L., Chio K. S. DNA-malonaldehyde reaction: formation of fluorescent products. Biochem Biophys Res Commun. 1972 Aug 21;48(4):921–926. doi: 10.1016/0006-291x(72)90696-1. [DOI] [PubMed] [Google Scholar]
- SASLAW L. D., ANDERSON H. J., WARAVDEKAR V. S. ULTRA-VIOLET PHOTOLYSIS OF UNSATURATED FATTY ACIDS IN RELATION TO THE THIOBARBITURIC ACID TEST. Nature. 1963 Dec 14;200:1098–1099. doi: 10.1038/2001098a0. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Shohet S. B. Remodeling of granulocyte membrane fatty acids during phagocytosis. J Clin Invest. 1974 Mar;53(3):726–734. doi: 10.1172/JCI107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Evaluation of opsonic and leukocyte function with a spectrophotometric test in patients with infection and with phagocytic disorders. Blood. 1973 Jul;42(1):121–130. [PubMed] [Google Scholar]
- Stossel T. P. Quantitative studies of phagocytosis. Kinetic effects of cations and heat-labile opsonin. J Cell Biol. 1973 Aug;58(2):346–356. doi: 10.1083/jcb.58.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Jacobs A. A., Sbarra A. J. Role of the Phagocyte in Host-Parasite Interactions XXVII. Myeloperoxidase-H(2)O(2)-Cl-Mediated Aldehyde Formation and Its Relationship to Antimicrobial Activity. Infect Immun. 1971 Apr;3(4):595–602. doi: 10.1128/iai.3.4.595-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966 Jun;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D. The percentage of monocytes among "mononuclear" cell fractions obtained from normal human blood. J Immunol. 1974 Jan;112(1):234–240. [PubMed] [Google Scholar]


