Abstract
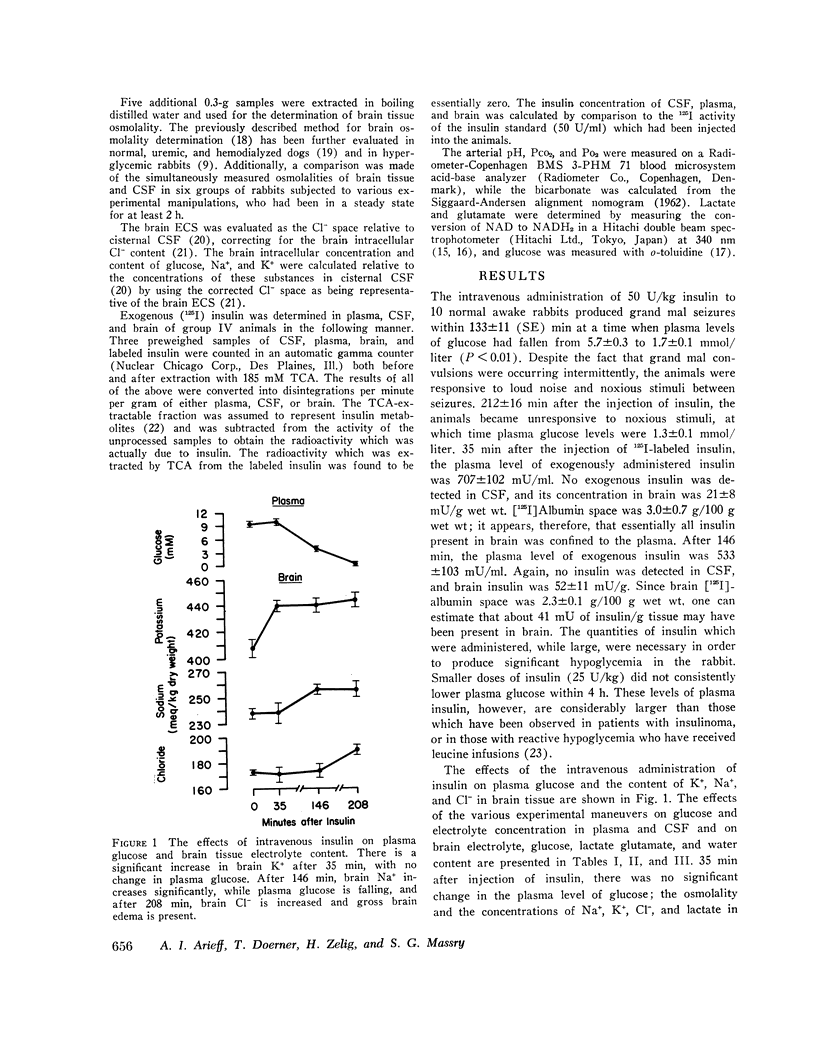
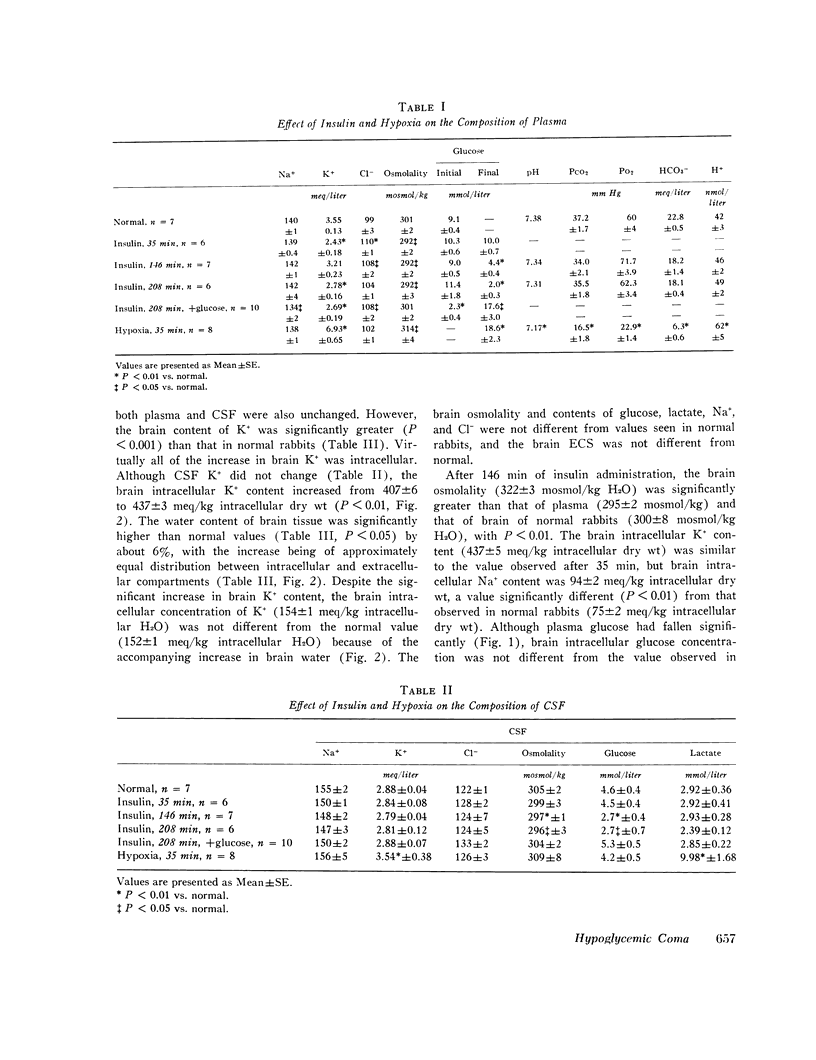
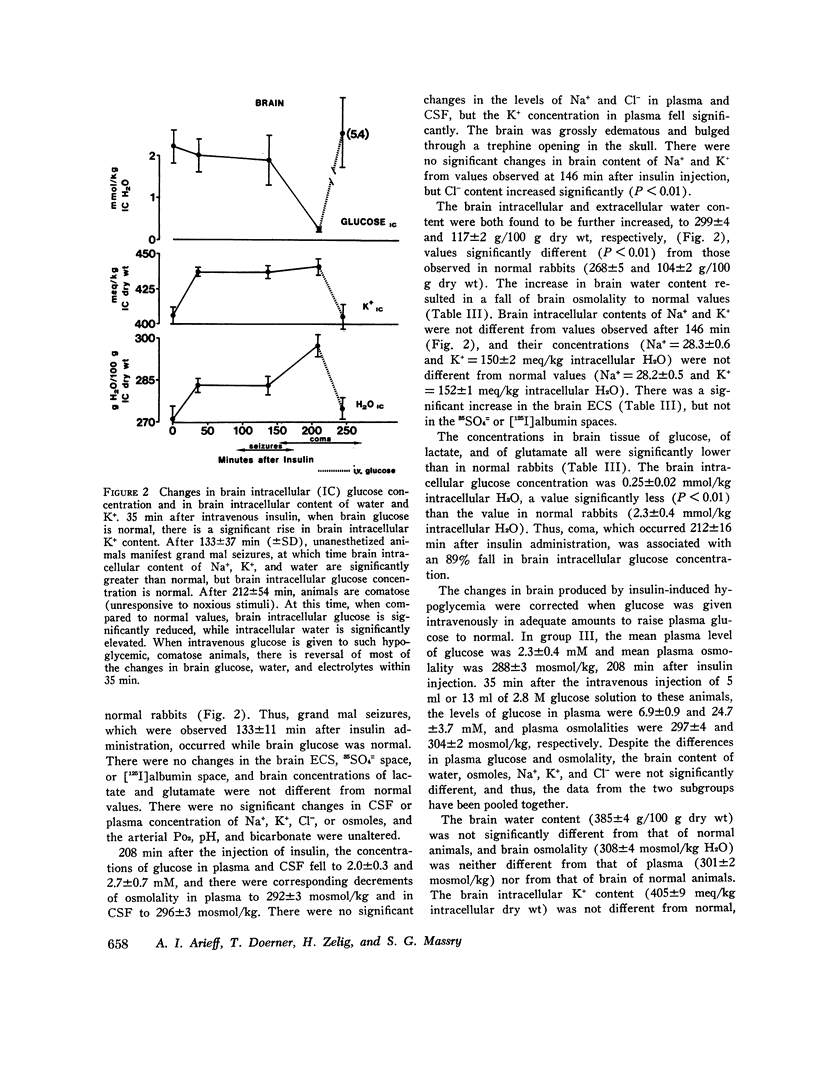
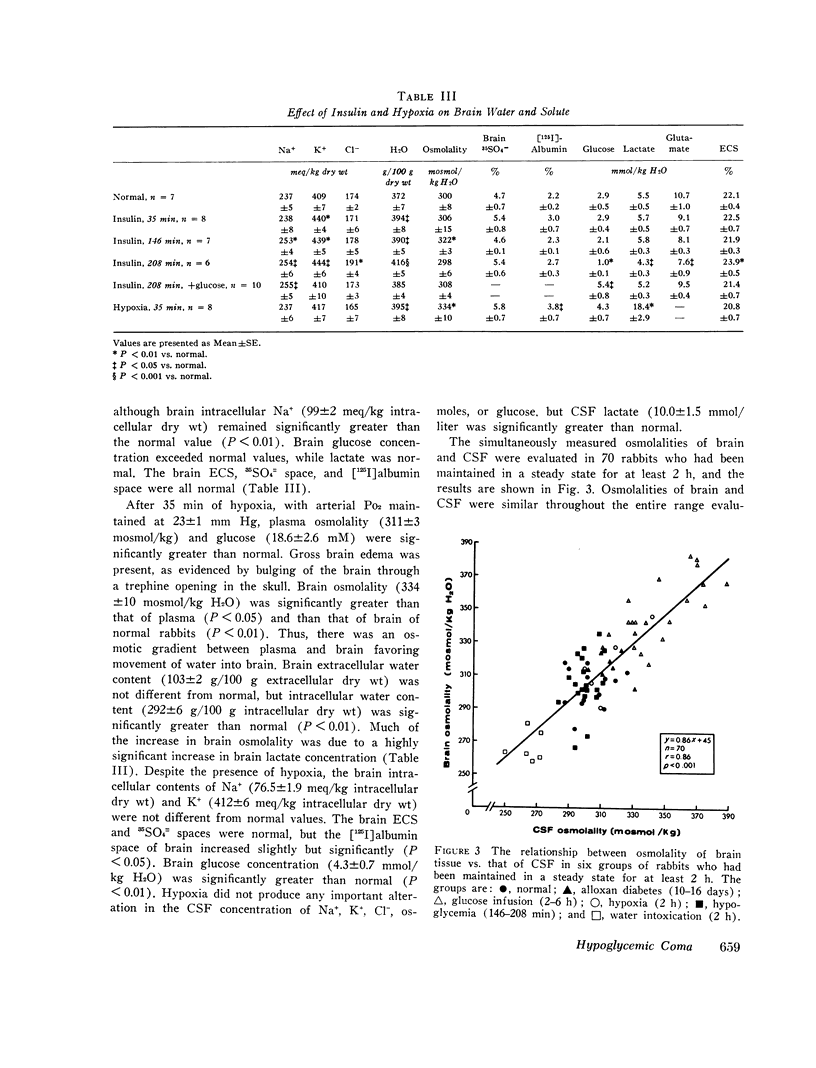
The mechanisms involved in the production of hypoglycemic coma were studied in rabbits. Measurements were made in brain, cerebrospinal fluid (CSF), and plasma of osmolality, Na+, K+, Cl-, water content, exogenous insulin, glucose, lactate, and glutamate, while pH, Pco2, Po2, and bicarbonate were evaluated in arterial blood, 35 min after i.v. injection of insulin (50 U/kg), plasma glucose did not change, but brain K+ content increased significantly. Grand mal seizures were observed in unanesthetized animals (±SD) 133±37 min after administration of insulin, at a time when brain glucose was normal, but brain tissue content of Na+, K+, osmoles, and water was significantly greater than normal. Coma supervened 212±54 min after insulin injection, at which time brain glucose, lactate, and glutamate were significantly decreased. At both 35 and 146 min after insulin administration, exogenous insulin was present in brain, but not in the CSF. After 208 min of insulin administration, animals were given i.v. glucose and sacrificed 35 min later. Most changes in the brain produced by hypoglycemia were reversed by the administration of glucose. Hypoxia (Po2 = 23 mm Hg) was produced and maintained for 35 min in another group of animals. Hypoxia caused brain edema but did not affect brain electrolyte content. However, brain lactate concentration was significantly greater than normal. The data indicate that the seizures noted early in the course of insulin-induced hypoglycemia are temporally related to a rise in brain osmolality secondary to an increased net transport into brain of Na+ and K+, probably caused by insulin, per se. As hypoglycemia persists, there is also depletion of energy-supplying substrates (glucose, lactate, glutamate) in the brain, an event which coincides with the onset of coma. The brain edema observed during hypoxia is largely due to an increase in brain osmolality secondary to accumulation of lactate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Nesbett F. B. Intracellular and extracellular compartments of mammalian central nervous tissue. J Physiol. 1966 May;184(1):215–238. doi: 10.1113/jphysiol.1966.sp007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieff A. I., Kleeman C. R., Keushkerian A., Bagdoyan H. Brain tissue osmolality: method of determination and variations in hyper- and hypo-osmolar states. J Lab Clin Med. 1972 Feb;79(2):334–343. [PubMed] [Google Scholar]
- Arieff A. I., Kleeman C. R. Studies on mechanisms of cerebral edema in diabetic comas. Effects of hyperglycemia and rapid lowering of plasma glucose in normal rabbits. J Clin Invest. 1973 Mar;52(3):571–583. doi: 10.1172/JCI107218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieff A. I., Massry S. G., Barrientos A., Kleeman C. R. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973 Sep;4(3):177–187. doi: 10.1038/ki.1973.100. [DOI] [PubMed] [Google Scholar]
- BARLOW C. F., DOMEK N. S., GOLDBERG M. A., ROTH L. J. Extracellular brain space measured by S35 sulfate. Arch Neurol. 1961 Jul;5:102–110. doi: 10.1001/archneur.1961.00450130104012. [DOI] [PubMed] [Google Scholar]
- Bell W. E., Samaan N. A., Longnecker D. S. Hypoglycemia due to organic hyperinsulinism in infancy. Arch Neurol. 1970 Oct;23(4):330–339. doi: 10.1001/archneur.1970.00480280044005. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Kleeman C. R. Stability of the potassium content of cerebrospinal fluid and brain. Am J Physiol. 1967 Aug;213(2):519–528. doi: 10.1152/ajplegacy.1967.213.2.519. [DOI] [PubMed] [Google Scholar]
- Brondsted H. E. Exchange of glucose between plasma, brain extracellular fluid and cerebral ventricles in cats and effects of intraventricular acetazolamide and insulin. Acta Physiol Scand. 1970 Sep;80(1):122–130. doi: 10.1111/j.1748-1716.1970.tb04776.x. [DOI] [PubMed] [Google Scholar]
- CLASEN R. A., COOKE P. M., PANDOLFIS, BOYD D., RAIMONDI A. J. Experimental cerebral edema produced by focal freezing. 1. An anatomic study utilizing vital dye techniques. J Neuropathol Exp Neurol. 1962 Oct;21:579–596. doi: 10.1097/00005072-196210000-00006. [DOI] [PubMed] [Google Scholar]
- COX R. W., HENLEY E. D., WELSH G. W., 3rd, WILLIAMS R. H. Insulin I-131 metabolism in man; plasma-binding, distribution and degradation. Am J Med. 1956 Sep;21(3):324–338. doi: 10.1016/0002-9343(56)90034-1. [DOI] [PubMed] [Google Scholar]
- Chowdhury F., Bleicher S. J. Studies of tumor hypoglycemia. Metabolism. 1973 May;22(5):663–674. doi: 10.1016/0026-0495(73)90238-2. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Prockop L. D., Winegrad A. I. Acute cerebral oedema during treatment of hyperglycaemia. An experimentsl model. Lancet. 1968 Aug 17;2(7564):384–386. doi: 10.1016/s0140-6736(68)90597-7. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- Duffy T. E., Nelson S. R., Lowry O. H. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem. 1972 Apr;19(4):959–977. doi: 10.1111/j.1471-4159.1972.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Chang M. M. Brain metabolism during hypoglycemia. Effect of insulin on regional central nervous system glucose and energy reserves in mice. Arch Neurol. 1973 Mar;28(3):173–177. doi: 10.1001/archneur.1973.00490210053006. [DOI] [PubMed] [Google Scholar]
- Gilboe D. D., Andrews R. L., Dardenne G. Factors affecting glucose uptake by the isolated dog brain. Am J Physiol. 1970 Sep;219(3):767–773. doi: 10.1152/ajplegacy.1970.219.3.767. [DOI] [PubMed] [Google Scholar]
- Herrera F. C. Effect of insulin on short-circuit current and sodium transport across toad urinary bladder. Am J Physiol. 1965 Oct;209(4):819–824. doi: 10.1152/ajplegacy.1965.209.4.819. [DOI] [PubMed] [Google Scholar]
- Hiatt N., Sheinkopf J. A. Treatment of experimental hyperkalemia with large dosages of insulin. Surg Gynecol Obstet. 1971 Nov;133(5):833–836. [PubMed] [Google Scholar]
- Kaasik A. E., Nilsson L., Siesjö B. K. The effect of asphyxia upon the lactate, pyruvate and bicarbonate concentrations of brain tissue and cisternal CSF, and upon the tissue concentrations of phosphocreatine and adenine nucleotides in anesthetized rats. Acta Physiol Scand. 1970 Apr;78(4):433–447. doi: 10.1111/j.1748-1716.1970.tb04680.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Levin E., Arieff A., Kleeman C. R. Evidence of different compartments in the brain for extracellular markers. Am J Physiol. 1971 Nov;221(5):1319–1325. doi: 10.1152/ajplegacy.1971.221.5.1319. [DOI] [PubMed] [Google Scholar]
- Levine R. The action of insulin at the cell membrane. Am J Med. 1966 May;40(5):691–694. doi: 10.1016/0002-9343(66)90149-5. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E. Effect of insulin on potassium transfer in isolated rat liver. Am J Physiol. 1961 Jun;200:1315–1319. doi: 10.1152/ajplegacy.1961.200.6.1315. [DOI] [PubMed] [Google Scholar]
- NELSON R. A., BEARGIE R. J. RELATIONSHIP BETWEEN SODIUM AND GLUCOSE TRANSPORT IN CANINE JEJUNUM. Am J Physiol. 1965 Feb;208:375–379. doi: 10.1152/ajplegacy.1965.208.2.375. [DOI] [PubMed] [Google Scholar]
- Norris J. W., Pappius H. M. Cerebral water and electrolytes. Effect of asphyxia, hypoxia, and hypercapnia. Arch Neurol. 1970 Sep;23(3):248–258. doi: 10.1001/archneur.1970.00480270058008. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Otsuki I. Mechanism of muscular paralysis by insulin with special reference to periodic paralysis. Am J Physiol. 1970 Nov;219(5):1178–1182. doi: 10.1152/ajplegacy.1970.219.5.1178. [DOI] [PubMed] [Google Scholar]
- Prockop L. D. Hyperglycemia, polyol accumulation, and increased intracranial pressure. Arch Neurol. 1971 Aug;25(2):126–140. doi: 10.1001/archneur.1971.00490020044005. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. II. THE INTERACTION BETWEEN ACTIVE SODIUM AND ACTIVE SUGAR TRANSPORT. J Gen Physiol. 1964 Jul;47:1043–1059. doi: 10.1085/jgp.47.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudek C. D., Boulter P. R., Knopp R. H., Arky R. A. Sodium retention accompanying insulin treatment of diabetes mellitus. Diabetes. 1974 Mar;23(3):240–246. doi: 10.2337/diab.23.3.240. [DOI] [PubMed] [Google Scholar]
- Senior B., Loridan L. Gluconeogenesis and insulin in the ketotic variety of childhoofd hypoglycemia and in control children. J Pediatr. 1969 Apr;74(4):529–539. doi: 10.1016/s0022-3476(69)80035-1. [DOI] [PubMed] [Google Scholar]
- Sloviter H. A., Yamada H. Absence of direct action of insulin on metabolism of the isolated perfused rat brain. J Neurochem. 1971 Jul;18(7):1269–1274. doi: 10.1111/j.1471-4159.1971.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Stone W. E., Tews J. K., Whisler K. E., Brown D. J. Incorporation of carbon from glucose into cerebral amino acids, proteins and lipids, and alterations during recovery from hypoglycaemia. J Neurochem. 1972 Feb;19(2):321–332. doi: 10.1111/j.1471-4159.1972.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Whittam R., Wheeler K. P. Transport across cell membranes. Annu Rev Physiol. 1970;32:21–60. doi: 10.1146/annurev.ph.32.030170.000321. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. DYNAMICS OF INSULIN SECRETION IN HYPOGLYCEMIA. Diabetes. 1965 Jun;14:341–349. doi: 10.2337/diab.14.6.341. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Effect of insulin on potassium efflux from rat muscle in the presence and absence of glucose. Am J Physiol. 1960 May;198:1066–1070. doi: 10.1152/ajplegacy.1960.198.5.1066. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L. Possible mechanisms of insulin action on membrane potential and ion fluxes. Am J Med. 1966 May;40(5):735–739. doi: 10.1016/0002-9343(66)90154-9. [DOI] [PubMed] [Google Scholar]



