Abstract
Objective:
We report a case of left adrenal schwannoma in a 62-year-old man, incidentally discovered on an abdominal computed tomography. It was successfully treated with laparoscopic adrenalectomy.
Methods:
On admission, no remarkable findings were recognized in the patient's blood and urine examination, including adrenal function. Laparoscopic left adrenalectomy was performed with the diagnosis of a nonfunctioning adrenal tumor.
Results:
Macroscopically, the tumor (45 mm × 30 mm, 60 g) arose from the medulla of the adrenal gland with a clear border distinguishing it from surrounding tissues. Histologically, the tumor consisted uniformly of spindle cells that were positive for S-100. The cortex was compressed but showed no atrophy. The diagnosis of adrenal schwannoma was made.
Conclusion:
Although an increasing number of adrenal incidentaloma have been identified with the recent advances in imaging techniques, only a few cases of schwannoma of the adrenal gland have been reported. We reviewed the cases reported previously in an attempt to reveal the characteristic features of this rare disease.
Keywords: Adrenal gland, Schwannoma, Laparoscopic surgery, Adreno-medullary tumor
INTRODUCTION
Schwannoma is a benign neurogenic tumor originating from Schwann cells, which also produce the myelin sheath that covers peripheral nerves. Schwannoma are most commonly found in cranial and peripheral nerves. Also, spinal nerves are often affected. However, schwannoma arising from the adrenal gland is an extremely rare entity; only 11 cases have been reported in the literature.1–10 Herein, we report a case of adrenal schwannoma, successfully treated with laparoscopic adrenalectomy and review the cases previously reported in an attempt to clarify the characteristic features of this rare disease.
CASE REPORT
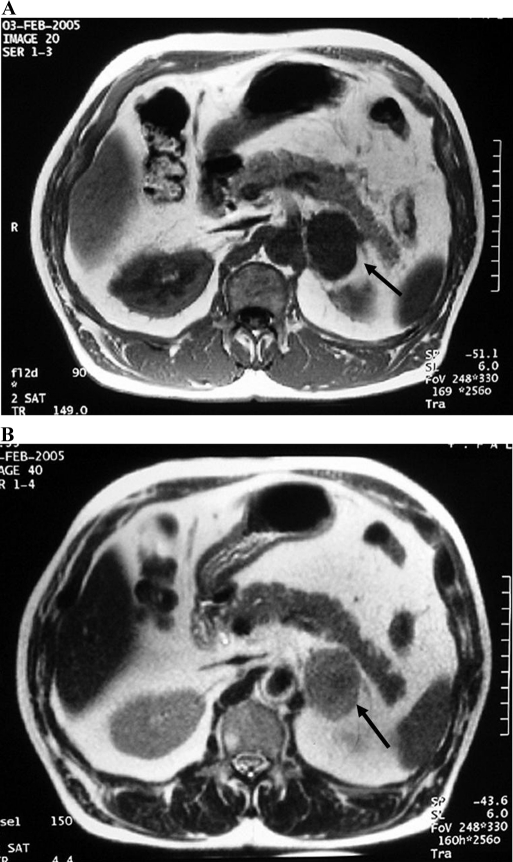
A 62-year-old man was referred to our hospital for further examination and treatment of his incidentaloma of the left adrenal gland. A local physician diagnosed the patient with mild liver dysfunction, and the patient had undergone an abdominal computed tomography as part of his workup. A round mass with a maximum diameter of 40 mm was recognized on the left adrenal gland. On admission, the patient was 161 cm tall and weighed 67 kg. His blood pressure was 132/92 mm Hg. No physical features were present to suggest Cushing's syndrome or von Recklinghausen's disease. The abdomen was soft and flat, and no abnormal mass was palpable. Magnetic resonance imaging (MRI) revealed a round mass 40 mm in diameter in the patient's left adrenal gland. The mass had a clear border setting it off from surrounding structures, and a portion of the mass had a lobular surface. The mass had low-signal intensity on a T1-weighted image and demonstrated slightly heterogeneous high-signal intensity on a T2-weighted image (Figure 1). Blood count and biochemistry were normal. Endocrinological examinations revealed no remarkable findings to suggest a functioning adrenal tumor (Table 1). Although ACTH was slightly elevated, it was thought to be nonspecific, because no alterations in either the morning level or the daily rhythm of cortisol secretion was observed. The diagnosis was made of a nonfunctioning left adrenal tumor. Because of the size and partly irregular shape of the mass, a laparoscopic transabdominal left adrenalectomy was performed to rule out a malignant tumor.
Figure 1.
Magnetic resonance imaging revealed a round mass in the left adrenal gland. The mass registered low-signal intensity on the T1-weighted image (A), and a slightly heterogeneously high-signal intensity on the T2-weighted image (B).
Table 1.
Endocrinological Data on Admission
| Plasma Levels (Normal Range) | ||
| ACTH | 62.3 pg/mL | (7.4-55.7) |
| Cortisol | 15.6 μg/dL | (4.0-18.3) |
| Rennin | 1.12 ng/ml/h | (0.2-2.7) |
| Aldosterone | 13.2 ng/dL | (2.0-13.0) |
| Dehydro epiandrosterone | 85 ng/mL | (13-264) |
| Adrenaline | 0.06 ng/mL | (0-0.17) |
| Nor-adrenaline | 0.29 ng/mL | (0.15-0.57) |
| Dopamine | 0.02 ng/mL | (0-0.03) |
| Urine Levels (Normal Range) | ||
| Dehydro epi-androsterone | 0.1 mg/day | (0-3.0) |
| Adrenaline | 7.3 μg/day | (1.0-23.0) |
| Noradrenaline | 75.5 μg/day | (29.0-120.0) |
| Dopamine | 340 μg/day | (100-1000) |
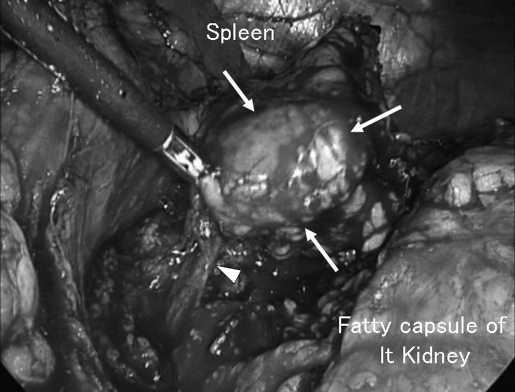
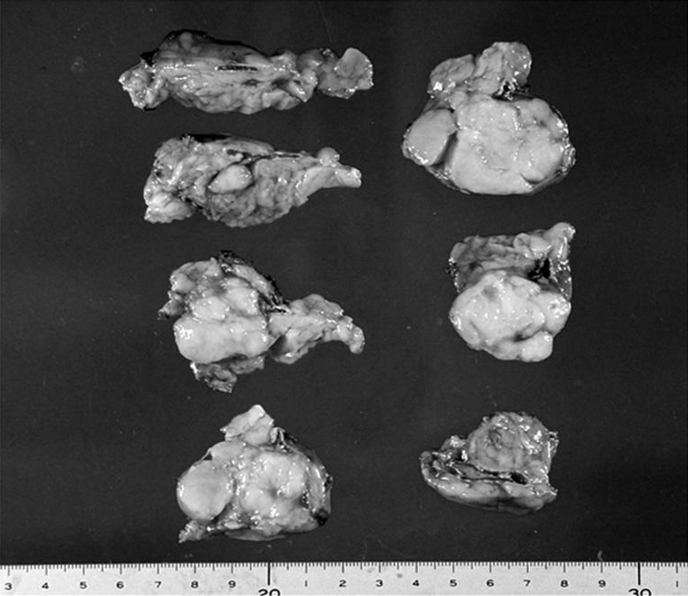
While under general anesthesia, the patient was placed in a right hemi-lateral position with a pillow under the right lower limb to lift up the left flank. An 11-mm trocar for a laparoscope was introduced by the open method at the level of 5 cm cranial to the navel on the left midclavicular line. After investigating the abdominal cavity to confirm no abnormal tumors, ascites, or adhesions, a 12-mm trocar for a vessel sealing system and 10-mm trocar for forceps were introduced under video vision at the left subcostal level on the anterior axillary line and on the median line, respectively. The spleen was mobilized by cutting the lieno-renal ligament, and the mass was easily found between the pancreas tail and the left kidney, surrounded by fatty tissue (Figure 2). The tumor with the adrenal gland was carefully isolated with sharp or blunt dissection, with surrounding fatty tissue, by using a vessel-sealing system. After the left adrenal vein was clipped and cut, preserving the left subphrenic vein, the isolated tumor was enclosed within an endoscopic pouch and was extracted via the extended wound of the 12-mm trocar. A clearly bordered round, firm mass was found within the left adrenal gland. The operation time was 86 minutes, and the blood loss was 100 g. The 45 mm × 30 mm × 30 mm tumor was observed to have developed from the medulla of the adrenal gland and weighed 60 g (Figure 3). Histologically, the tumor consisted of spindle cells without atypia or mitosis. A thin, fibrous area indistinctly separated the tumor from the surrounding nonatrophic adrenal cortex. Immunohistochemical analysis revealed that the tumor cells were uniformly positive for S-100, and partly positive for myeline, but were negative for c-kit, smooth muscle actin, CD34, and desmin. Thus, the tumor was diagnosed as schwannoma arising from the adrenal medulla. The patient was discharged uneventfully on the fifth day after surgery. Abdominal ultrasonography confirmed no recurrent disease, and the patient is in good health without disease 36 months after surgery.
Figure 2.
Laparoscopic view during the left adrenalectomy. The tumor (arrows) was removed with left adrenal gland and surrounding fatty tissues. Central vein (arrow head) was clipped and cut.
Figure 3.
Macroscopic view of the tumor that arose from the adrenal medulla, compressing the normally developed cortex.
DISCUSSION
The majority of the tumors originating in the adrenal medulla are pheochromocytoma, neuroblastoma, or ganglioneuroma. Schwannoma of the adrenal gland is rarely encountered, although it similarly originates from the cells of the neural crest. The origin of the adrenal schwannoma is considered to be either of 2 myelinized nerve systems innervated to the adrenal medulla. One is the sympathetic nerve from the upper lumbar plexus, and the other is the phrenic or vagus nerve.2 All reported cases demonstrate that the schwannoma originated in the medulla; no schwannoma has been reported to arise from the cortex. This is likely the consequence of the nerve in the adrenal cortex developing quite poorly compared with that in the medulla and only a few thin nerves running along the vasculature.
The diagnosis of schwannoma of the adrenal gland was difficult to make based on the imaging studies. Typical MRI findings report solid tumors with low-signal intensity on T1-weighted images and heterogeneously high-signal intensity on T2-weighted images, with occasional cystic components. However, imaging findings were nonspecific for the tumors with a nerve origin, such as neuroblastoma or ganglioneuroma.9 Every reported case has been treated as a nonfunctioning incidentaloma of the adrenal gland, and the diagnosis of schwannoma was determined postoperatively through histological examination.
Conventionally, schwannoma are divided into 2 distinct subtypes, hyper-cellular “Antoni A” type and hypo-cellular “Antoni B” type. Spindle cells are arranged in fascicles in areas of high cellularity with little stromal matrix in the Antoni A type. Nuclear-free zones called “Verocay bodies” can also be found in between the regions of nuclear palisading. Tumor cells are less densely found, forming loose meshwork often accompanied by microcysts or myxoid changes in Antoni B type, and are considered to be a degenerated form of the Antoni A type.2 Both sub-types can be found as a mixture in adrenal schwannoma; 3 of 11 reported cases demonstrated both subtypes in a tumor. The differential diagnosis made using immunostaining should exclude gastrointestinal stromal tumor (GIST) or leiomyoma. Schwannoma positively stains for S-100. By contrast, GIST is positive for c-kit, and leiomyoma is positive for smooth muscle actin.
Eleven cases of adrenal schwannoma have been previously reported1–10 in English and Japanese (Table 2) as far as we can determine from a search of PubMed and Japana Centro Revuo Medicina. There has been no age or sex predominance. Three patients experienced abdominal pain. The tumors in these 3 patients were large in size (75 mm, 90 mm, and 124 mm). In addition, a histological demonstration of a large cystic tumor5 and 2 small tumors incidentally found in the autopsy samples8 have been reported. Excluding these 5 cases, 7 adrenal schwannomas, including our case, were incidentally found by imaging analyses performed as a part of the workup for the nonspecific symptoms. The size ranged from 28 mm to 90 mm (average, 56) when identified, which was not considered large enough to cause clinical symptoms. Cases of malignant schwannoma have also been reported.11,12 They were demonstrated to be large solid masses (60 mm and 180 mm) extending over the adrenal gland occupying the retroperitoneum, and with unfavorable outcomes. Hori et al13 reported an extremely rare case of retroperitoneal schwannoma secreting catecholamine. Miettinen et al11 reported the coexistence of pheochromocytoma with malignant schwannoma. However, no case of catecholamine-secreting “benign” adrenal schwannoma has been reported. As in our case and another,6 slight elevation of ACTH was observed. However, these elevations were considered nonspecific because no evidence was present of alteration in cortisol secretion in either case. Thus, features of adrenal schwannoma should be considered the same as so-called typical “adrenal incidentaloma.”
Table 2.
Reported Cases of Adrenal Schwannoma
| # | Author | Year | Age | Sex | Symptom | Endocrine Abnormality | Laterality | Diameter | Weight | Solid or Cystic | Antoni Type | Operation Approach |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bedard | 1986 | 63 | F | Abdominal pain | None | Left | 90 mm | 180 g | Solid | ND* | Thoraco-abdominal |
| 2 | Watanabe | 1986 | 62 | M | Incidental (US*) | None | Right | 63 mm | 79 g | Solid | A & B | Lumbar |
| 3 | Shimada. | 1991 | 49 | F | Incidental (US*) | None | Right | 50 mm. | 60 g | Solid & cystic | A & B | Abdominal |
| 4 | Nezasa | 1992 | 14 | F | Abdominal pain | None | Left | 75 mm | 115 g | Solid | A | Abdominal |
| 5 | Lack | 1997 | 29 | F | ND* | ND* | Left | 140 mm | ND* | Cystic | A | ND* |
| 6 | Igawa | 1998 | 45 | M | Incidental (UGI) | Slight elevation of ACTH | Left | 65 mm | 75 g | Solid | A | Lumbar |
| 7 | Pittasch | 2000 | 56 | F | Abdominal pain | None | Right | 124 mm | ND* | Solid | A & B | ND* |
| 8 | Arena | 2004 | 89 | M | Incidental (Autopsy†) | ND* | Right | 10 mm | ND* | Solid | A | Autopsy |
| 9 | Arena | 2004 | 67 | M | Incidental (Autopsy‡) | ND* | Right | 6 mm | ND* | Solid | A | Autopsy |
| 10 | Suzuki | 2007 | 33 | M | Incidental (US) | None§ | Right | 90 mm | ND* | Solid | A | ND* |
| 11 | Korets | 2007 | 70 | M | Hematuria | None | Left | 28 mm | 65 g | Solid | A | Laparoscopic |
| 12 | Onoda | 2008 | 62 | M | Incidental (CT*) | None | Left | 45 mm | 60 g | Solid | A | Laparoscopic |
ND=not described; US=abdominal ultrasonography; UGI=upper gastrointestinal series; CT: computed tomography.
Died of pulmonary embolism.
Died of myocardial infarction
No abnormal findings with either 123I-metaiodobenzylguanidine nor 131I-6b-iodomethyl-19-nor-cholest-5(10)-en-3b-ol scintigraphy,
Incidentally observed asymptomatic tumor in the adrenal gland, incidentaloma, has been increasing, along with the advance in the field of imaging technology. These incidentalomas are most frequently cortical adenomas followed by carcinoma, pheochromocytoma, and myelolipoma. Intimate hormonal and imaging analyses can identify most of these major adrenal tumors.14 When excluding these major tumors by hormonal and imaging analysis, adrenal schwannoma should be expected, although it has been extremely rare. A current consensus in managing adrenal incidentalomas has been proposed.15 However, no well-established recommendation for surgical application has been developed for patients with tumors between 4 cm and 6 cm. The consensus suggests that criteria in addition to size should be considered in making the decision to monitor or proceed to adrenalectomy. Relevant variables to perform laparoscopic adrenalectomy for incidentaloma have been proposed that include a low complication rate (less than 3%) and the informed consent.16
CONCLUSION
The present case suggests that application of laparoscopic adrenalectomy to the incidentaloma could be a feasible choice, when performed by an experienced surgical team with appropriate informed consent, not only as a minimally invasive treatment, but also as a whole tumor biopsy to establish a correct diagnosis.
Contributor Information
Naoyoshi Onoda, Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan..
Tetsuro Ishikawa, Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan..
Takahiro Toyokawa, Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan..
Tsutomu Takashima, Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan..
Kenichi Wakasa, Department of Diagnostic Pathology, Osaka City University Graduate School of Medicine, Osaka, Japan..
Kosei Hirakawa, Department of Surgical Oncology, Osaka City University Graduate School of Medicine, Osaka, Japan..
References:
- 1.Bedard YC, Horvath E, Kovacs K. Adrenal schwannoma with apparent uptake of immunoglobulins. Ultrastruct Path. 1986;10:505–513 [DOI] [PubMed] [Google Scholar]
- 2.Watanabe Y, Nishikido M, Kubota S, et al. Neurinoma arising from the adrenal gland: A case report [in Japanese with English abstract]. Nishi Nippon Hinyoki. 1986;48:543–547 [Google Scholar]
- 3.Shimada K, Kobayashi T. A case of adrenal schwannoma [in Japanese]. Hinyoki Geka. 1991;4:1207–1210 [Google Scholar]
- 4.Nezasa S, Horie M, Kobayashi S, Shinoda T. Adrenal schwannoma in child: A case report [in Japanese with English abstract]. Rinnsho Hinyokika. 1992;46:963–965 [Google Scholar]
- 5.Lack EE. Other unusual primary adrenal tumors. In: Atlas of Tumor Pathology 19. Tumors of the Adrenal Gland and Extra-Adrenal Paraganglia. Washington, DC: Armed Forces Institute of Pathology; 1997 [Google Scholar]
- 6.Igawa T, Hakariya H, Tomonaga M. Primary adrenal schwannoma [in Japanese]. Nippon Hinyokika Gakkai Zasshi. 1998;89:567–570 [DOI] [PubMed] [Google Scholar]
- 7.Pittasch D, Klose S, Schmitt J, et al. Retroperitoneal schwannoma presenting as an adrenal tumor. Exp Clin Endocrinol Diabetes. 2000;108:318–321 [DOI] [PubMed] [Google Scholar]
- 8.Arena V, DeGiorgio F, Drapeau CMJ, Monego G, DeMercu-rio D, Capelli A. Adrenal schwannoma. Report of two cases. Folia Neuropathol. 2004;42:177–179 [PubMed] [Google Scholar]
- 9.Suzuki K, Nakanishi A, Kurosaki Y, Nogaki J, Takaba E. Adrenal schwannoma: CT and MRI findings. Radiat Med. 2007;25:299–302 [DOI] [PubMed] [Google Scholar]
- 10.Korets R, Berkenblit R, Ghavamian R. Incidentally discovered adrenal schwannoma. JSLS. 2007;11:113–115 [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen M. Pheochromocytoma combined with malignant schwannoma: Unusual neoplasm of the adrenal medulla. Ultra-struct Path. 1988;12:513–527 [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa H, Imai T, Noguchi T, et al. Malignant schwannoma. A report of three cases [in Japanese with English abstract]. Shinshu Med. 1988;36:533–541 [Google Scholar]
- 13.Hori T, Yamagiwa K, Yagi S, et al. Noradrenalin-secreting retroperitoneal schwannoma resected by hand-assisted laparoscopic surgery: report of a case. Surg Today. 2006;36:1108–1113 [DOI] [PubMed] [Google Scholar]
- 14.Mantero F, Terzolo M, Arnaldi G, et al. A survey on adrenal incidentaloma in Italy. J Clin Endocrinol Metab. 2000;85:637–644 [DOI] [PubMed] [Google Scholar]
- 15.NIH State-of-the-Science Statement on management of the clinically inapparent adrenal masses (“incidentaloma”). NIH Consensus State Sci Statements. 2002;19(2):1–23 [PubMed] [Google Scholar]
- 16.Brunaud L, Kebebew E, Sebag, et al. Observation or laparoscopic adrenalectomy for adrenal incidentaloma? A surgical decision analysis. Med Sci Monit. 2006;12:355–362 [PubMed] [Google Scholar]