Abstract
Cilia are complex structures that have garnered interest because of their roles in vertebrate development and their involvement in human genetic disorders. In contrast to multicellular invertebrates in which cilia are restricted to specific cell types, these organelles are found almost ubiquitously in vertebrate cells, where they serve a diverse set of signaling functions. Here, we highlight properties of vertebrate cilia, with particular emphasis on their relationship with other subcellular structures, and explore the physiological consequences of ciliary dysfunction.
Cilia are polarized structures that appeared early in the evolution of eukaryotes. Although initially studied for their roles in cell and/or fluid propulsion, it is now known that cilia serve a variety of sensory functions in both unicellular and multicellular organisms. This expanding view of ciliary roles is coincident with an increased appreciation of both their structural complexity and their content diversity. Whereas original estimates placed the number of proteins at the basal body and ciliary axoneme at 150 and 250, respectively (Dutcher, 1995), the recent assessment of ten comparative genomics and proteomics data sets enriched for basal body and ciliary proteins suggests that the vertebrate cilium may require up to ~1000 different polypeptides for its function (Gherman et al., 2006, and http://www.ciliaproteome.org). Integration of an additional proteomics study reporting the protein content of the connecting cilium and outer segment of the mouse photoreceptor potentially augments this number even further (Liu et al., 2007a). Yet, it is not clear what minimal fraction of those proteins is required in situ as opposed to other cellular sites. The projected complexity of the total ciliary protein complement reflects the multifaceted roles that cilia play in vertebrates. Here, we provide a perspective of our current understanding of ciliary biology, highlighting examples of ciliary roles and exploring the consequences of ciliary dysfunction.
Ciliary Structure in Vertebrates
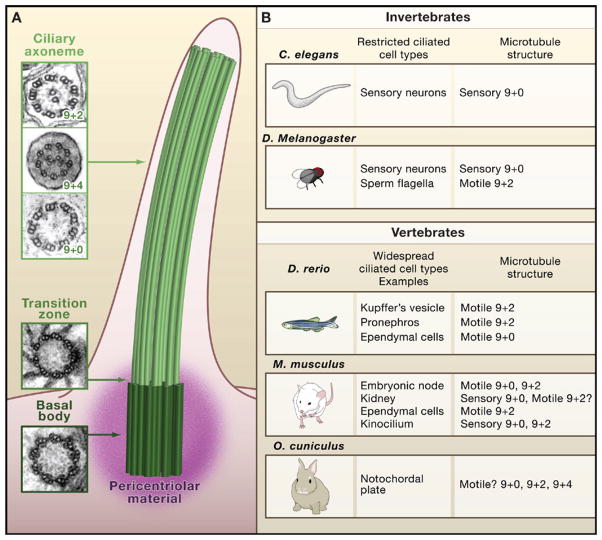
Much of our understanding of ciliary constituents is based on observations made from flagella isolated from the green alga Chlamydomonas (Dutcher, 1995). The archetypical cilium extends from the apical membrane of cells and relies on a microtubule scaffold (Figure 1A). Surrounded by a plasma membrane, the ciliary axoneme begins as a radial array of microtubule triplets at its cellular anchor, the basal body. The common ciliated eukaryotic ancestor also contained a set of inner microtubules, known as the “central pair,” which has either been maintained during evolution in the “9+2,” or lost in the “9+0” configurations. Although the majority of motile cilia possess the 9+2 arrangement, and most nonmotile cilia have the 9+0 configuration, notable exceptions include motile 9+0 ependymal cilia in the central canal of the zebrafish spinal cord (Kramer-Zucker et al., 2005; Figure 1B) or immotile 9+2 cilia in frog olfactory epithelium (Reese, 1965).
Figure 1. Ciliary Structure in Vertebrates.
(A) Microtubule structure of the vertebrate cilium. Cilia are tethered to the apical aspect of the cell at the basal body, surrounded by pericentriolar material. Nine radially organized microtubule triplets protrude from the basal body to the transition zone, and then extend as microtubule doublets in the ciliary axoneme. Electron micrograph images of each portion of the cilium are shown to the left. Images are from Kramer-Zucker et al. (2005) and Feistel and Blum (2006), reprinted with permission from John Wiley & Sons, Inc. from Developmental Dynamics, Vol. 235, 2006, p. 3348–3358. Copyright (2006) John Wiley & Sons, Inc., and Fromherz et al. (2004), reproduced with permission from Journal of Cell Science.
(B) Diversification of ciliary architecture in vertebrate species. Whereas invertebrate models such as C. elegans and D. melanogaster have cilia restricted to certain cell types, nearly all vertebrate cells are ciliated. Examples shown from zebrafish, mouse, and rabbit demonstrate the possible expansion of ciliary roles in vertebrates. This expansion is exemplified by populations of cilia in vertebrate tissues that are architecturally heterogeneous, such as the rabbit notochordal plate and the mouse kinocilium. Moreover, there are exceptions to the general association between the central microtubule pair and motile cilia, such as motile ependymal cilia (9+0) in the zebrafish spinal cord and nonmotile sensory kinocilia (9+2) in the mouse inner ear.
Intraflagellar Transport
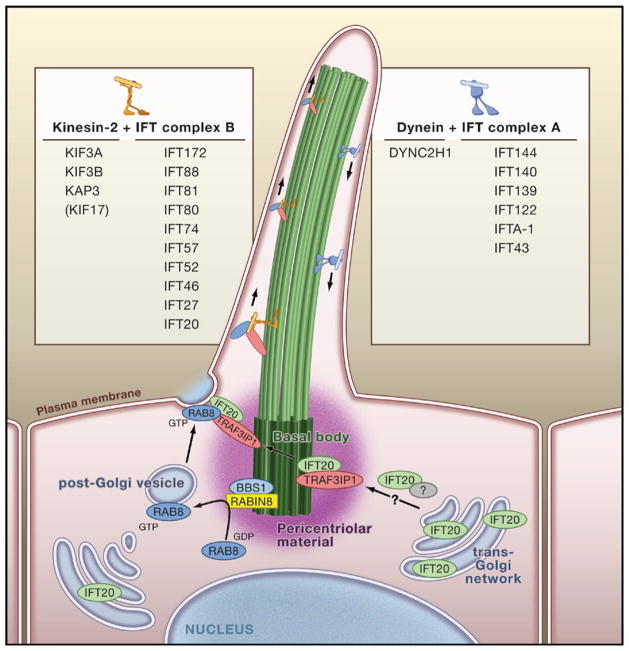
Our current comprehension of intraflagellar transport (IFT) is based on experiments carried out in Chlamydomonas and subsequently in C. elegans (for a recent review, see Scholey, 2008). Given that protein synthesis is not known to occur at the cilium, proteins travel to their final destination by the dynamic cooperation of molecular motors and protein scaffolds. Anterograde IFT is mediated by the heterotrimeric kinesin-2 motor, and cytoplasmic dynein facilitates retrograde IFT. In cooperation with these motors, “core” protein complexes known as IFT particles A (retrograde) and B (anterograde) are thought to bind to protein cargo through protein interaction domains and in vertebrates the complexes consist of at least six and ten different proteins respectively (Scholey, 2008) (Figure 2).
Figure 2. Vesicular Transport and Intraflagellar Transport.
Accumulating evidence indicates that vesicular transport of ciliary proteins from the trans-Golgi network is critical for proper cilium formation and may be facilitated, at least in part, by the intraflagellar transport (IFT) particle protein IFT20. Furthermore, Rabin8 interacts directly with Bardet-Biedl Syndrome protein BBS1, complexed with the BBSome in the basal body. Rabin8 is required for Rab8 GDP/GTP exchange. Rab8-GTP is proposed to be transported in vesicles to the base of the cilium, where it may dock with the ciliary membrane and/or the IFT complex. Upon targeting to the base of the cilium, ciliary proteins are transported toward the ciliary tip with the kinesin-2 motor in cooperation with IFT complex B proteins (listed on the left; shown in orange). TRAF3IP1/elipsa has been shown to function as an accessory anterograde IFT protein that binds directly with IFT20. Recycling of proteins toward the basal body is mediated by a cytoplasmic dynein and IFT complex A proteins (listed on the right; shown in blue).
A combination of biochemical and in vivo studies on IFT mutants has catalyzed the discovery of a number of critical roles for IFT and cilia in general with regard to vertebrate physiology (see http://v3.ciliaproteome.org/pdf/Vertebrate%20ciliary%20mutants.pdf). For example, the cooperative sensory and motile roles of cilia in the embryonic node in mouse and the Kuppfer’s vesicle in zebrafish, which are abolished in a variety of IFT mutants, have highlighted the critical role of cilia in the early events in left-right axis determination (Hirokawa et al., 2006; Tabin, 2006). Likewise, defects in either IFT particle assembly or receptor targeting to the ciliary membrane are linked to defective intracellular Ca2+ responses to mechanical stimuli (Yoder, 2007), and the biophysical properties of cilia are important in the dynamics of ciliary motility (Marshall and Kintner, 2008). As each of these topics have been reviewed in depth recently, the following sections focus on recent advances and newly emerging areas of ciliary research.
Cell Biology of Primary Cilia
Cilia, Golgi, and Vesicular Transport
Emerging clues suggest that cilia have specific, and potentially physiologically relevant, interactions with other intracellular compartments. One of the cellular organelles found consistently in the proximity of the primary cilium and the basal body is the Golgi apparatus. In the 1960s, the availability of electron microscopy led to the observation that the Golgi network localizes close to the basal body, and ciliogenesis follows the flattening of a vesicle to the distal end of the centriole destined to form the basal body of the newly formed cilium (Sorokin, 1964). In later stages of ciliogenesis, the ciliary sheath forms out of the initial primary ciliary vesicle (Sorokin, 1964). When electron microscopy was complemented with confocal laser scanning microscopy, the characterization of spatial relationships became more accessible. In chondrocytes and aortic smooth muscle cells, the primary cilium, the Golgi, and the nucleus are consistently found in proximity to each other (Poole et al., 1997), which has since been observed in other cell and tissue types, likely highlighting a functional, but as yet unknown, link between these organelles.
The relationship between the primary cilium and Golgi along with sequence homology between IFT proteins and coat protein I (COPI) and clathrin-coated vesicle components (Jekely and Arendt, 2006) suggest evolutionary roots of the primary cilium in vesicle transport. Indeed, the structural correlation between the primary cilium and the Golgi is exemplified by the localization of Xklp3, a Xenopus subunit of the kinesin-2 complex, on vesicles of the intermediate compartment (IC) between the endoplasmic reticulum and the Golgi apparatus (LeBot et al., 1998). This suggested relationship has been highlighted more recently: IFT20, a component of the IFT particle B complex, localizes to both the Golgi and the primary cilium (Follit et al., 2006) (Figure 2). The role of IFT20 is unlikely to overlap with kinesin-2 because, at least in human retinal pigment epithelial (RPE) cells, IFT20 depletion suppresses ciliary assembly but does not affect either the integrity of the Golgi complex or the localization of kinesin-2 on Golgi vesicles. Nonetheless, the question still remains whether these data reflect two independent (one ciliary, one Golgi-specific) or interlinked roles for this protein.
A series of observations have begun to assemble a model in which vesicular transport (perhaps from the trans-Golgi network) is necessary for ciliogenesis and potentially also important for protein targeting to the transition zone of mature cilia. The zebrafish coiled-coil polypeptide elipsa is a binding partner of Ift20 and Rabaptin5, a regulator of endocytosis, which in turn interacts with Rab8, a small membrane-associated GTPase (Omori et al., 2008). Notably, Rab8 is known to participate in rhodopsin transport in photoreceptors by regulating docking and fusion of rhodopsin-bearing post-Golgi membranes (Moritz et al., 2001), and loss of Rab8 results in an accumulation of vesicles at the base of the photoreceptor connecting cilium. Moreover, Rab8a has been shown to bind directly to cenexin/ODF2, a centrosomal and basal body component (Yoshimura et al., 2007). In addition, the recently identified BBSome, a complex of seven of the fourteen known causative Bardet-Biedl Syndrome (BBS) proteins, provides more evidence for a ciliary role of Rab8, as it is thought to bind to the C terminus of Rabin8, the guanosyl exchange factor specific for Rab8 (Nachury et al., 2007).
Cell Polarity and Ciliogenesis
The primary cilium is a polarized structure. It is therefore not surprising that several independent observations now support an intimate link between the primary cilium and the establishment and maintenance of cell polarity.
The formation of cell and tight junctions is important for cellular polarization and the subsequent formation of polarized structures such as the cilium; polarity complexes localize to cellular junctions, where several basal body and ciliary components are observed as well, such as inversin (Nurnberger et al., 2002) (Figure 3A). The reverse is also true, in that several factors determining cell polarity localize to both the tight junctions and the primary cilium, such as the complex consisting of the partitioning-defective protein 3 (Par3), partitioning-defective protein 6 (Par6), and atypical protein kinase Cζ (aPKCζ) (Fan et al., 2004) (Figure 3A). However, it is not clear whether these data indicate a functional link between cell and tight junctions or unrelated functions of the proteins dependent on their cellular context.
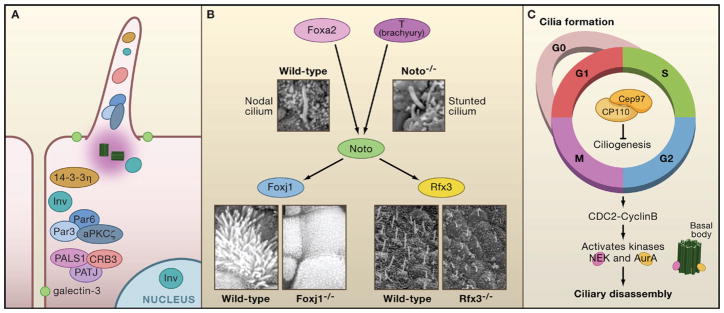
Figure 3. Control of Ciliogenesis.
(A) Ciliary proteins localize to cell junctions and polarity proteins localize to the primary cilium. Inversin localizes to three separate cellular protein pools: the primary cilium, cell junctions, and the nucleus. Two different polarity complexes, Par3/Par6/aPKCζ and CRB3/PATJ1/PALS, as well as the polarity protein 14-3-3η, localize to cell junctions and the primary cilium. Galectin-3 also marks a membrane region of increased lipid density surrounding the cilium.
(B) The transcription factors Noto, FoxJ1, and Rfx3 play a role in ciliogenesis. Noto acts downstream of Foxa2 and T (brachyury) and upstream of FoxJ1 and Rfx3. Embryonic node and nodal cilium in E7.5 wild-type and Noto-deficient mice (Beckers et al., 2007) reveal structurally abnormal and stunted cilia. Images are reproduced with permission from PNAS, Vol. 104, p. 15765–15770. Copyright (2007) National Academy of Sciences, U.S.A. Scanning electron micrographs of explanted trachea show multiciliated cells covering most of the apical surface in wild-type trachea. These are absent from mice that are Foxj1 deficient, whereas primary cilia are unaffected. Scanning micrographs of embryonic nodes of Theiler stage 11c of wild-type and Rfx3 deficient mice. Cilia in Rfx3 deficient mice are shorter and misoriented compared to the wild-type (Bonnafe et al., 2004). Images reproduced from Molecular and Cellular Biology, 2004, Vol. 24, p. 4417–4427, with permission from the American Society for Microbiology.
(C) A complex of Cep97 and CP110 suppresses ciliogenesis throughout the cell cycle. Mitotic entry is regulated by the G2/M transition through CDC2-Cyclin B, which activates several kinases, including the Nek family and AurA. In murine inner medullary collecting duct (IMCD3) cells, NEK5 localizes to the centrosome and the distal tip of the basal body. In human retinal pigment epithelial cells, a pool of AurA localizes to the basal body.
Does the primary cilium actively participate in the establishment of apical-basolateral polarity, or is it the outcome of such a process? As an apical structure, it is most likely a product of the polarization process. Consistent with this notion, perturbations in the polarizing processes result in defects in ciliogenesis. Loss of a component of the apical transport machinery, phosphatidylinositol-4-phosphate adaptor protein 2 (FAPP2) leads to delayed cilia formation in adherent Madin-Darby canine kidney (MDCK) cells and abolishes cilia formation in MDCK cysts (Vieira et al., 2006). In FAPP2-depleted cells, tight junctions are fully functional, but localization of galectin-3, a subapical marker, indicates a polarity defect. Moreover, loss of FAPP2 leads to a transient accumulation of subapical transport vesicles in the vicinity of the microtubule-organizing center and to a shift in the balance of apical and basolateral membrane lipid rafts. Subsequently, an RNA interference (RNAi) screen in MDCK cells identified several candidate apical transport proteins involved in ciliogenesis or regulation of ciliary length (Torkko et al., 2008).
The establishment of cell polarity in epithelial cells thus appears to be a prerequisite for ciliogenesis, and apical transport proteins fulfill dual roles in cell polarization and cilia assembly. It is important to note, however, that mesenchymal cells are ciliated even though they lack apicobasal polarity. On the other hand, the presence of the cilium might itself exert an effect in maintaining cell polarity. Disruption of ciliary components such as KIF3A or polaris/IFT88 leads to cell polarity defects: suppression of Kif3a leads to both loss of cell polarity and defective ciliogenesis in vitro (Fan et al., 2004; Lin et al., 2003). In kidneys of mice with a hypomorphic mutation in Tg737/Ift88, the localization of the epidermal growth factor receptor (EGFR), which in cells of wild-type mice is typically basolateral, extends into the apical membrane of renal cysts (Taulman et al., 2001). Taken together, these data suggest several alternatives. First, we cannot exclude the possibility that proteins such as KIF3A have nonciliary roles, disruption of which leads to the observed polarity defects. For example, KIF3A also plays a role in spindle formation and mitosis (Haraguchi et al., 2006). At the same time, it is reasonable to speculate that the loss of the primary cilium can lead to the impairment of the establishment or maintenance of apical polarity. It will be interesting, in this regard, to examine the behavior, assembly, and stability of the Par3/Par6/aPKCζ complex in a series of ciliary mutants, particularly those that have functional but not overt structural defects in ciliary assembly and maintenance.
Ciliary Dynamics
Centrosomes are the anchoring structures for both cilia, in the form of the basal body, and mitotic spindles. Although basal bodies and centrosomes on one hand and mitotic spindles and centrosomes on the other can easily interconvert, there are no reports of cells with both cilia and mitotic spindles. In fact, primary cilia are regarded as postmitotic structures of quiescent cells. Ciliary dynamics thus appear to be precisely coordinated with cell cycle progression; the molecular cues triggering ciliary assembly and disassembly, however, are understood insufficiently.
The known cues for ciliary assembly seem to act through derepression, indicating that the cellular default state would be active ciliary assembly: a complex of two centrosomal proteins, Cep97 and CP110, likely acts as a suppressor of ciliogenesis (Spektor et al., 2007); depletion of either Cep97 or CP110 in mammalian cells leads to mislocalization of the other binding partner and either monopolar or multipolar spindle pole formation. The concurrent suppression of Cep97 and CP110 messages results in assembly of cilia-like structures containing well-established ciliary markers such as glutamylated and acetylated tubulin, polaris/IFT88, and polycystin-2 (PC2), as well as centriole markers including centrin in cells that had not exited the cell cycle (Figure 3C). Because centrin is contained over the entire length of the structures, it has been argued that these are not cilia but rather elongated centrioles (Keller et al., 2008). In addition, the increased presence of binucleated cells suggests a cytokinesis defect likely connected to aberrant mitotic spindle formation. Overexpression of CP110 in serum-starved quiescent cells suppressed the formation of primary cilia without perturbation of cellular quiescence, further indicative of a repressive function on ciliogenesis (Spektor et al., 2007). During G0, CP110 is not expressed, indicating that ciliation might be initiated through a yet unknown mechanism triggering transcriptional repression of CP110.
Recent evidence suggests the presence of a CP110 population complexed by CEP290/NPHP6 that is separate from other complexes involved in cell cycle progression or ciliation, such as Cep97/CP110 (Tsang et al., 2008). CEP290 localizes to the centrosome and both daughter and mother centrioles, but does not seem to play a role in centrosome function, because cell cycle progression and spindle formation are unaffected by its loss. However, interaction of CP110 and CEP290 is required for suppression of ciliary assembly, indicating that the centrosomal CEP290 population may be targeted to the structure that will give rise to the basal body. Yet, depletion of CEP290 leads to loss of ciliogenesis (Graser et al., 2007), indicating that further experiments are needed to resolve the functions of CEP290.
At the other end of the ciliary life cycle, retraction of the primary cilium and re-entry into mitosis are tightly coordinated, and the details of this process are beginning to be illuminated. Ciliary disassembly is not a prerequisite for cell cycle entry, because loss of CP110 leads to ciliary formation in nonquiescent cells (Spektor et al., 2007). Intriguingly, two of the kinases that regulate mitotic entry are involved in retracting the cilium, suggesting that the G2/M transition is tightly coordinated with ciliary disassembly (O’Connell et al., 2003): In Chlamydomonas reinhardtii, a genetic screen for deflagellation mutants identified FA2, encoding a member of the Nek family of kinases, which plays a role both in basal body/centriole associated microtubule severing and in cell cycle progression. Interestingly, in mice, mNek1, mNek8, and mNek5/Fa2p are associated with the basal body and centrioles in a cell cycle-dependent manner (Mahjoub et al., 2005) (Figure 3C), and perturbations in Nek1 and Nek8 cause polycystic kidneys in mice and zebrafish (Liu et al., 2002; Upadhya et al., 2000).
Another kinase that regulates ciliary disassembly is Aurora A (AurA), a homolog of Chlamydomonas aurora/Ipl1p-like protein kinase (CALK) (Pan et al., 2004; Pugacheva et al., 2007). Interaction of AurA with scaffolding protein HEF-1 at the basal body activates AurA and triggers ciliary disassembly through phosphorylation/activation of histone deacetylase 6 (HDAC6), a microtubule deacetylase (Pugacheva et al., 2007) (Figure 3C), suggesting that ciliogenesis and ciliary disassembly are tightly coordinated with, but are not integral components of, the cell cycle. Although the cilium is a characteristic of quiescent cells, it does not inhibit mitosis, and disruption of the cilium does not lead to widespread proliferative phenotypes. The precise mechanism by which cilium disassembly is synchronized to cell cycle progression will have to be addressed in more detail, particularly the time at which it occurs. One hypothesis is that CDC2-CyclinB directly activates retraction of the primary cilium (O’Connell et al., 2003).
Transcriptional Regulation of Ciliation
Ciliogenesis is an intricate, coordinated process involving dozens, and possibly hundreds, of proteins. Studies in Chlamydomonas have demonstrated that a host of genes are upregulated after deflagellation (Li et al., 2004). However, little is known about the ciliogenesis circuit in vertebrates, or indeed the regulatory processes that might specifically regulate the various architectural and functional types of vertebrate cilia.
The homeobox gene Noto regulates the formation of nodal cilia downstream of Foxa2 and T (Abdelkhalek et al., 2004). Mice lacking Noto show defects in the caudal notochord and laterality, varying from heterotaxy to situs inversus or situs solitus. Although cilia are present in the posterior notochord, they are both reduced in number and shorter in length, with partially disorganized or incomplete microtubular structures (Beckers et al., 2007) (Figure 3B) In addition, expression levels of the transcription factors Rfx3 and FoxJ1, each of which is critical to ciliogenesis, are reduced or completely abolished, suggesting that Noto acts upstream of both factors (Beckers et al., 2007). Mice deficient in Rfx3 show left-right asymmetry defects (Bonnafe et al., 2004) (Figure 3B), and a C. elegans ortholog of Rfx3, daf-19, regulates the expression of a number of ciliary genes (Efimenko et al., 2005; Li et al., 2004). Likewise, mice deficient in Foxj1 die either in utero or in the early neonatal period because of hydrocephalus or heterotaxia, and they lack respiratory cilia (Brody et al., 2000; Chen et al., 1998). Subsequent studies have shown that Foxj1-deficient mice have primary 9+0 cilia but lack motile 9+2 cilia, implicating FoxJ1 in motile but not primary ciliogenesis, although it is not clear whether the primary cilia function normally (Zhang et al., 2004) (Figure 3B). Recently, the role for FoxJ1 in motile cilia formation has been expanded from mice to Xenopus and zebrafish (Stubbs et al., 2008; Yu et al., 2008). This suggests that overall ciliary architecture is regulated and maintained by similar transcriptional mechanisms regardless of cilia subtypes, because Noto, which controls length, number, and microtubular structure of all cilia, acts upstream of FoxJ1, which specifically regulates formation of 9+2 cilia.
Although we know some of the key players in the transcriptional activation of ciliogenesis, we do not know the triggering events involved in cilia formation. One possible exception, at least for the formation of motile cilia in multiciliated cells, is the implication of a role for notch3 and jagged2 in the regulation of cell fate in the epithelial tubules of the zebrafish pronephros (Liu et al., 2007b; Ma and Jiang, 2007). Suppression of either signaling component expands ciliary gene expression and leads to a greater number of multiciliated cells in the pronephros, whereas ectopic expression of notch1a represses the differentiation of multiciliated cells.
The Primary Cilium in Signal Transduction
Regulation of Canonical and Noncanonical Wnt Signaling
In addition to apicobasal polarity, cells also establish a plane of polarity distinguishing proximal from distal ends, a process regulated by planar cell polarity (PCP) signaling. In vertebrates, PCP signaling represents a subset of noncanonical Wnt signaling, and hallmarks of defective noncanonical Wnt signaling include impaired convergent extension movements during gastrulation, neural tube closure failure, and disorganization of stereocilia in the mouse inner ear (Veeman et al., 2003). Extracellular Wnt stimulates the signal transduction cascade by binding to a complex of the Frizzled (Fz) receptor and the low-density lipoprotein receptors LRP5 or LRP6. In humans, there are 19 Wnt ligands, ten Fz receptors, and numerous Wnt effectors, acting in a context- and tissue-dependent manner (Figure 4A). The signal is transmitted by Disheveled (Dvl), which localizes to two separate pools within the cell depending on the signaling context. Nuclear localization of Dvl is necessary for the β-catenin-dependent or canonical Wnt signaling pathway, which results in transcriptional activation of genes under the control of T cell-specific transcription factor/lymphoid enhancer factor-1 (TCF/LEF-1)-responsive elements (Itoh et al., 2005). In the context of noncanonical Wnt signaling, Dvl is commonly localized to the plasma membrane (Axelrod et al., 1998; Rothbacher et al., 2000; Seto and Bellen, 2004).
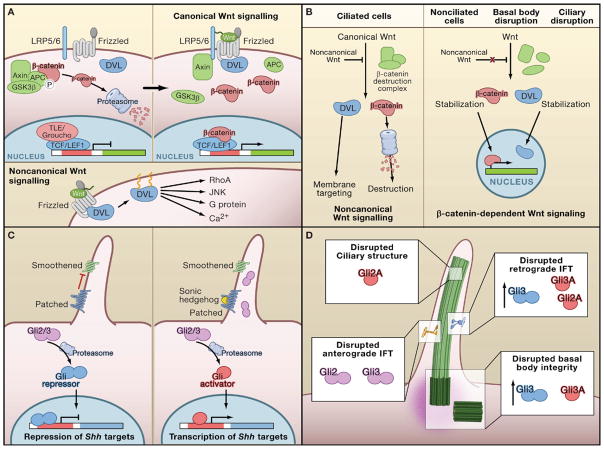
Figure 4. The Primary Cilium in Wnt and Shh Signaling.
(A) In the absence of Wnt stimuli, β-catenin is constitutively phosphorylated by the β-catenin destruction complex consisting of Axin, APC, and GSK3β. Phosphorylated β-catenin is targeted for degradation. Wnt targets are repressed by a complex of TCF/LEF1 and TLE. In canonical Wnt signaling, the ligand binds to a complex of the Frizzled receptor and LRP5/6 coreceptor, which then binds to Axin and Dishevelled (DVL), leading to stabilization of β-catenin in the cytoplasm. β-catenin migrates into the nucleus, replaces TLE, and activates transcription ofβ-catenin/TCF/LEF1-responsive genes. In noncanonical signaling, activated DVL is targeted to the membrane and activates downstream targets.
(B) Effects of ciliary and basal body disruption on Wnt signaling. In ciliated cells, noncanonical Wnt signals antagonize canonical Wnt signaling, leading to proteasomal β-catenin degradation and membrane targeting of DVL. In unciliated cells, canonical Wnt signaling stabilizes cytoplasmic β-catenin and DVL, leading to increased nuclear levels of both DVL and β-catenin, initiating transcription of TCF/LEF1-responsive genes. Disruption of ciliary or basal body components leads to loss of noncanonical Wnt signaling and stabilization of both DVL and β-catenin in the cytoplasm and nucleus, resembling the status of nonciliated cells.
(C) In the absence of sonic hedgehog (Shh) ligand, Patched (Ptch) represses Smoothened (Smo). Repressive forms of the Gli proteins bind to the promoter regions of Shh targets and inhibit transcription. Stimulation with Shh ligand recruits Smo and Ptch1 to the primary cilium. Gli2 and Gli3 are localized to both the cilium and the nucleus, and in response to the ligand stimulus they are proteasomally processed into their activator Gli2/3A isoforms, activating transcription of Shh targets.
(D) Differential role of ciliary and basal body proteins in Gli2/3 processing. Perturbation of anterograde intraflagellar transport (IFT) leads to loss of Gli2 and Gli3 processing and Shh signaling. Loss of structural integrity of the primary cilium by disruption of Arl13b leads to constitutively active Gli2, but leaves Gli3 unaffected. Loss of retrograde IFT triggers both Gli2 and Gli3 proteasomal activation along with transcriptional upregulation of Gli3. Finally, disruption of the basal body does not affect Gli2 processing but increases both expression and proteasomal activation of Gli3.
Several studies have implicated ciliary and basal body proteins in the regulation of Wnt signaling: Inversin (Inv) interacts with Dvl and targets the cytoplasmic fraction of Dvl for degradation (Simons et al., 2005). Suppression of Xinv in Xenopus embryos results in defects in convergent extension, suggesting that Inversin acts as a switch required for noncanonical Wnt signaling and suppression of β-catenin activity. Although these experiments showed that Inv regulates the balance of canonical and noncanonical Wnt signaling, it was not clear which cytoplasmic population of Inv—separate protein pools are associated with the cilium, adherens junction, and nucleus—would be involved. The BBS proteins, most of which localize specifically to the basal body, provide further insight. Both mouse and zebrafish BBS models manifest symptoms typical of defective PCP signaling. Moreover, studies in Bbs models show a genetic interaction of BBS genes with core PCP genes, suggesting that basal bodies are involved in PCP signaling (Gerdes et al., 2007; Ross et al., 2005). Concordant with the implication of Inversin in the balance between canonical and noncanonical Wnt signaling (Simons et al., 2005), zebrafish bbs morphants show both defects in convergent extension indicative of impaired noncanonical Wnt signaling and expanded expression domains of β-catenin transcriptional targets. Depletion of BBS proteins in vitro results in an augmented response to Wnt3a (Gerdes et al., 2007).
Another study investigated the role of three different ciliary and basal body proteins in β-catenin-dependent Wnt signaling: Kif3a, Ift88, and Ofd1 (Corbit et al., 2008). Loss of Kif3a in mice leads to enhanced β-catenin-dependent transcriptional activation on embryonic day E9.0. Embryonic fibroblasts from both Kif3a and orpk mutant mice, as well as Ofd1-deficient embryonic stem cells (see http://v3.ciliaproteome.org/pdf/Vertebrate%20ciliary%20mutants.pdf), are hyperresponsive to Wnt3a through the canonical Wnt pathway.
Both studies reveal an inhibitory role of the primary cilium on the canonical Wnt signaling pathway. Loss of ciliary or basal body components leads to hyperresponsiveness to external canonical Wnt stimuli (Corbit et al., 2008; Gerdes et al., 2007) at the expense of noncanonical Wnt signaling (Gerdes et al., 2007) (Figure 4B).
Accumulating evidence also suggests some planar polarity components are necessary for ciliogenesis. Suppression of Inturned (Xint) and Fuzzy (Xfy) during Xenopus development affects ciliogenesis, and the observed Shh and PCP signaling defects of Xint and Xfy morphants are consistent with the general lack of cilia (Park et al., 2006). In Xint-depleted cells, microtubular structures resembling ciliary axonemes are formed, but the actin network orienting and organizing the centrioles as the ciliary base is missing. Given that int and fy were identified originally as genes in Drosophila that encode planar polarity effector proteins (Adler et al., 2004), the lack of ciliogenesis in Xint and Xfy morphants could implicate a role of planar polarity signaling in centriole migration and thus indirectly in ciliogenesis. However, although core PCP polarity proteins are required for the asymmetric localization of other planar polarity proteins, Inturned and Fuzzy do not affect the asymmetric localization of core PCP proteins (Adler et al., 2004). The reciprocity between ciliary function and noncanonical Wnt signaling is also reflected in the targeted disruption of Ift88 in the developing mouse cochlea, causing misorientation of kinociliary basal bodies and thus stereociliary bundles, whereas core PCP proteins localize normally (Jones et al., 2008). This suggests that ciliary and basal body integrity are critical for the correct translation of polarized, morphogenetic cues.
Evidence for an involvement of the PCP signaling pathway in ciliogenesis comes from zebrafish duboraya (dub) morphants, which show defects both in primary ciliary assembly and left-right patterning (Oishi et al., 2006). Importantly, the dub phenotype is replicated by suppression of frizzled-2 message, and suppression of the noncanonical Wnt signaling pathway is characterized by a misorganization of the actin skeleton (Oishi et al., 2006). The potential conflict between the involvement of cilia for the balance between canonical and noncanonical Wnt signaling pathway on one hand and the requirement of noncanonical Wnt signaling for proper ciliogenesis on the other will likely be resolved when the actual signaling cascades in a time- and tissue-specific context are identified.
Hedgehog Signaling
In mammals, the family of Hedgehog (Hh) signaling proteins consists of three different pathways governed by Indian hedgehog (Ihh), Desert hedgehog (Dhh), and Sonic hedgehog (Shh) ligands. The Hh ligand binds to the Patched (Ptch) receptor (two in mammals), which then releases repressed Smoothened (Smo) and by doing so activates downstream signaling components, including the Glioma (Gli) proteins (three in mammals) (Lum and Beachy, 2004; Hedgehog Signaling Database, http://hedgehog.sfsu.edu) (Figure 4C).
Several independent lines of evidence have demonstrated a role of the primary cilium in Hh signaling. Initially, a screen for modulators of mouse embryonic patterning identified two mutants, wimple (wim) and flexo (fxo), with characteristic Shh defects, including neural tube closure defects, abnormal brain morphology, and preaxial polydactyly (Huangfu et al., 2003). The ethylnitrosourea (ENU)-induced mutations were mapped to two IFT genes, Ift172 (wim) and Ift88/polaris (fxo), and a third ciliary transport mutant deficient for Kif3a had similar defects in floor plate specification; all three mutants showed reduced Shh signaling. Subsequently, Smo was found to localize to the primary cilium in the mouse embryonic node, which is involved in both left-right determination and floor plate induction (Corbit et al., 2005). Upon Shh stimulation, both Smo and Ptch1 are recruited to the cilium both in vitro and in vivo, where Ptch1 binds to Shh and then leaves the cilium, possibly by internalization (Rohatgi et al., 2007). Downstream effectors of the Hedgehog signaling pathway, Gli2 and Gli3, also localize to the primary cilium of mesenchymal and ectodermal cells of the developing limb bud (Haycraft et al., 2005). Disruption of IFT88/polaris leads to loss of function of both Gli2 and Gli3, suggesting a role of the cilium in processing and function of Gli2 and Gli3, but not Gli1, which activates target genes normally in IFT88-deficient cells.
Several lines of evidence suggest a differential role of ciliary/basal body proteins in Gli2/3 processing (Figure 4D). First, mouse mutants with disrupted IFT particle B genes (such as Ift52, Ift57, and Ift88) (Haycraft et al., 2005; Houde et al., 2006; Huangfu et al., 2003; Liu et al., 2005) exhibit phenotypes characteristic of loss of Shh signaling implicating anterograde IFT in proper Smo/Ptch1 localization. Second, in hennin (hnn) mutant mice, which harbor a mutation in the gene encoding the Arf-family GTPase Arl13b (Caspary et al., 2007), and whose cilia are shortened and have a defect in the axoneme, Gli3 repressor activity is unaltered compared to wild-type mice. However, Gli2 and possibly Gli3 are active ectopically, albeit at low levels, indicating that ciliary architecture plays a role in Gli2, but not necessarily Gli3, processing and function (Caspary et al., 2007). Third, the alien (aln) mouse, harboring a null mutation in the retrograde transport protein Ttc21b/Ift139, shows signs of excessive Shh signaling (Tran et al., 2008). A role for retrograde IFT in Gli3 processing (Huangfu and Anderson, 2005; May et al., 2005) and Gli2 activity might explain the observed overactivity of both Gli2 and Gli3 in aln mice. Finally, in Ftm knockout mice, Gli3 processing is specifically affected, suggesting a role for basal body proteins in Hedgehog signaling, specifically Gli3 processing (Vierkotten et al., 2007).
The availability of conditional ciliary mutants has facilitated the dissection of the role of Shh signaling in a variety of different tissue types and organisms. Hh signaling in skeletogenesis has been evaluated by the conditional ablation of Kif3a in mouse cartilage (Koyama et al., 2007) and the conditional ablation of Kif3a or Ift88 in the developing limb (Haycraft et al., 2007), exposing a unique role of cilia and Ihh signaling in skeletogenesis, although the cartilage phenotype is only partially phenocopied by Ihh ablation (Koyama et al., 2007).
Similarly, targeted disruption of Kif3a in a subset of neural progenitors in mice led to reduced production of new neurons in the dentate gyrus, probably due to a lack of granule neuron precursors (Han et al., 2008). The phenotypic overlap between these mice, Ift88 hypomorphs, and Ftm-deficient mice suggests a general requirement for basal body and cilia function in proliferation of granule neuron precursors in the dentate gyrus. In addition, the observed proliferation defects coincided with decreased Shh activity in the developing brain at E18 as judged by both Gli1 and Ptch1 expression. Similar effects were observed in cerebrellar granule cell precursors, extending the role of Shh signaling beyond the dentate gyrus (Spassky et al., 2008). Thus, targeted ablation of primary cilia can identify novel roles for Hh signaling during development and adult tissue homeostasis.
With the role of the primary cilium in Hh signaling firmly established, it remains unclear whether all or only a subset of Hh signaling events are mediated by the primary cilium. The predominance of floor plate specification, neural tube ventralization, and limb patterning defects observed in ciliary mutants over other Hh-associated defects has been striking. The most severe ciliary mutant phenotypes (mice lacking Kif3a or Ift88) manifest in embryonic lethality at or around E10.5, preceding the developmental stages when Shh defects such as cyclopia (E12.5) or hair follicle maturation defects (E14.5) can be observed. Of note, Hh signaling components are highly conserved among Drosophila and mammals, although the pathway is active in nonciliated cells in the flies. It is unclear, however, whether this implicates a ciliary specialization in vertebrates and that the cilium has been co-opted by morphogenetic signaling pathways, or whether the cilium has been lost in the evolution of diptera such as Drosophila melanogaster. A more detailed understanding of the individual pathways in a time- and tissue-specific context will likely lead to a more nuanced view of the role of the primary cilium in Hedgehog signaling.
Other Signaling Pathways
In primary mouse embryonic fibroblasts and NIH 3T3 fibroblasts, platelet-derived growth factor receptor PDGFRαα as well as an activated downstream component (phospho- Mek1/2) localize to the primary cilium during G0 (Schneider et al., 2005). Embryonic fibroblasts of orpk mutant mice are not activated by PDGF-AA stimulation, implying that ciliary localization of PDGFRαα is necessary for proper signal transduction. While this is suggestive of a cilia-specific role for PDGFRα in homeostasis and mitogenic signaling, it has not yet been shown that PDGFRαα signaling regulates cell cycle reentry of quiescent cells in vivo.
G protein-coupled receptors also localize to rodent neuronal cilia, including the serotonin subtype 6 receptor (5-HT6) (Brailov et al., 2000), somatostatin receptor3 (Sstr3) (Handel et al., 1999), and melanin-concentrating hormone receptor1 (Mchr1) (Berbari et al., 2008). The loss of Bbs2 or Bbs4 leads to mislocalization of Sstr3 and Mchr1 in mice (Berbari et al., 2008). The localization of β-arrestin1/2 to the cilium may have implications beyond the Shh signaling pathway, where it mediates complex formation between Smo and Kif3a (Kovacs et al., 2008). This is because β-arrestins are known to play a role in the regulation of seven-transmembrane receptor-mediated signaling (Lefkowitz et al., 2006). Yet, it has not been demonstrated that ciliary localization of the receptors or β-arrestin is a prerequisite for somatostatin- or Mch-mediated signaling or that these signaling pathways are impaired in ciliary mutants.
Consequences of Ciliary Dysfunction
The Ciliopathies: Clinically Distinct but Overlapping Phenotypes
The link between pathological phenotypes and ciliary dysfunction in humans was proposed initially in the 1970s, when Bjorn Afzelius and others noticed that individuals with chronic bronchitis, immotile sperm, and situs inversus had diminished ciliary motility due to the lack of outer dynein arms (Afzelius, 1976). However, it was not until recently when defects in proteins that localize to the basal body and axoneme of nonmotile 9+0 primary cilia were causally related to human disease. With the help of complete genomic sequence information, coupled to both spontaneously mutated and gene-targeted vertebrate animal models, a multitude of human diseases have now been attributed to defects in ciliary and basal body proteins. Collectively, these human syndromes are termed “ciliopathies” (see Badano et al., 2006, and references within), disorders defined by unique clinical criteria, but which present many overlapping phenotypes such as retinal degeneration, polydactyly, situs inversus, mental retardation, encephalocele (due to failure of neural tube closure), and cysts in the kidney, liver, and pancreas. We proposed previously that these common phenotypes could be used as predictors to implicate the genes/proteins involved in disorders of unknown genetic basis in ciliary dysfunction, and a recent report demonstrating that mutations in IFT80 are associated with Jeune Asphyxiating Thoracic Dystrophy (JATD) represents a proof of principle for this idea (Beales et al., 2007).
In the following section, rather than exploring each ciliopathy in detail (for comprehensive reviews, see Badano et al., 2006; Fliegauf et al., 2007), we will highlight cellular and molecular explanations for the phenotypic variation both within and across syndromes.
The Cellular Basis of Pleiotropy
Given that cilia are present in most vertebrate tissues, it is not surprising to observe pleiotropy in ciliary disorders. However, the extent to which patients are affected (expressivity) depends on the spatiotemporal context of the mutated protein. Some ciliopathies, such as autosomal dominant polycystic kidney disease (ADPKD), and the earlier-onset autosomal recessive polycystic kidney disease (ARPKD) are largely confined pathologically to the kidneys, liver, and pancreas. This might be attributed to tissue-specific expression of the mutated gene products, which are membrane-associated proteins that localize to the cilium, such as polycystin-1 (PC1), polycystin-2 (PC2) (ADPKD), and fibrocystin/polyductin (ARPKD). These are thought to mediate the balance between proliferation and differentiation during organogenesis (Nauli et al., 2003; Wang et al., 2004). On the other hand, global basal body and ciliary disorders are caused by compromised protein function in multiple tissues. For example, Bardet-Biedl (BBS), Meckel-Gruber (MKS), and Joubert Syndrome (JBTS) patients all share overlapping phenotypes such as cystic kidneys, central nervous system (CNS) developmental defects, and polydactyly (Badano et al., 2006). Accordingly, all genes that cause these three disorders are necessary for proper function of the basal body and cilium during development in a wide range of tissues (Table S1, available online).
Tissue specificity and temporal expression of ciliary proteins is only one facet adding to our understanding of the complexity of ciliary phenotypes. An additional contributing factor is the observation that some proteins that carry out distinct functions in cilia may localize to two discrete cellular pools, which may either be functionally related or be devoted to unique cellular tasks. PC2 is a notable example of a ciliary protein that carries out dual cellular roles. Nauli and colleagues first proposed that in primary cell cultures of mouse renal cilia, PC2 functions in conjunction with PC1 as an ion channel required for fluid-flow-induced Ca2+ signaling (Nauli et al., 2003). Additionally, a cilia-specific role for PC2 in Ca2+ signaling, albeit independently of PC1, has also been demonstrated in the mouse node; McGrath et al. observed that PC2 localizes to both peripheral and central monocilia in the node at E7.5 and that asymmetric calcium transients correspond to the presence of PC2 (McGrath et al., 2003). However, PC2 has also been shown to function as a Ca2+ channel at the plasma membrane and endoplasmic reticulum (Tsiokas et al., 2007). Intriguingly, cardiovascular abnormalities are a major complication of ADPKD, which may result from dysfunctional PC2-mediated Ca2+ signaling at the sarcoplasmic membrane of smooth muscle cells (Qian et al., 2003). This represents a potential ciliary-independent phenotype of ADPKD, although the paucity of information about ciliary function in muscle cells does not merit exclusion of a possible ciliary role for PC2 in the cardiovascular system.
Inversin is another protein contributing to renal pathologies that may be conducting multiple roles within the cell. We know from the inv mouse model that inversin plays a role in both the node in the establishment of left-right asymmetry (reviewed by Hirokawa et al., 2006), and also in the promotion of renal development and homeostasis. Reports of inversin localization to the intercellular cell membrane (Nurnberger et al., 2002), coupled with data demonstrating that inversin is expressed as early as the two-cell stage in mouse embryos, presents the possibility that inversin may function prior to the appearance of the node to contribute in a yet unknown way to establish left-right asymmetry (Eley et al., 2004). Studies in Xenopus embryos have demonstrated that left-right asymmetry may be established before the development of Spemann’s Organizer (the mouse node equivalent) with differential cell membrane potentials governed by H+/K+ ATPase expression (Levin et al., 2002). Therefore, we cannot exclude the possibility that inversin functions similarly in a nonciliary role to break left-right asymmetry.
The cilia-independent role of KIF3A in establishing polarity in neurons has complicated the assessment of the true ciliary phenotype in null models. Abrogation of Kif3a in mice pheno-copies other IFT complex mutants; they are embryonic lethal and display laterality defects resulting from the absence of cilia at the node (Marszalek et al., 1999). However, KIF3A was recently reported to function as a KIF3B-independent molecular motor that interacts with the PAR3/PAR6/aPKCζ complex to establish neuron polarity. Two independent reports showed that KIF3A is the molecular motor required to transport PAR3 to the tip of developing hippocampal neurites to specify the neuronal axon and, consequently, neuronal polarity (Nishimura et al., 2004; Shi et al., 2004). As a preliminary attempt to explore the central nervous system in ciliary mutants, Kif3a was conditionally targeted in the mouse cerebellum and the observed abnormal development was attributed in part to defects in granule cell proliferation (Chizhikov et al., 2007). However, it remains to be elucidated whether altered neuronal polarity might contribute to the phenotype in these mutants.
In contrast to PC2, inversin, and KIF3A, which carry out similar functions in different cellular compartments, IFT57/Hippi (huntingtin interacting protein-1 protein interactor) performs two entirely different roles in the cell. IFT57 is an IFT complex B protein, and mice lacking Hippi display laterality defects and aberrant Shh downregulation consistent with the absence of cilia (Houde et al., 2006). However, Hippi has been documented previously as a contributor to the neuronal apoptosis observed in Huntington’s disease (Gervais et al., 2002). These observations challenge the notion that some of the neuronal degenerative phenotypes that we observe in ciliopathy patients are due exclusively to ciliary function, particularly if IFT57 is responsible for any of the pathogenic or modifying alleles that we observe in ciliary syndromes.
Allelic Variants: Same Gene, Differing Severity
In agreement with the substantial number of proteins that are required for correct function of the basal body and cilium in vertebrates, we know that ciliopathies are not only pleiotropic in the phenotypes they present, but they are also oligogenic, reflecting the importance of each component of protein complexes that are functioning at the basal body and cilium (Table S1). Importantly, there are now several reports suggesting that pathogenic mutations in one particular gene can contribute to clinically distinct ciliary disorders as allelic variants manifested along a phenotypic spectrum of severity.
On the basis of the phenotypic overlap between MKS, JBTS, BBS, and nephronophthisis (NPHP), several groups have been prompted to examine causative genes across different ciliopathy cohorts. For example, variants in BBS2, BBS4, and BBS6 have been identified in MKS or MKS-like fetuses (Karmous-Benailly et al., 2005). MKS3 mutations have been associated with JBTS (Baala et al., 2007b), and mutations in RPGRIP1L have been reported in MKS, JBTS, and NPHP patients (Arts et al., 2007; Delous et al., 2007; Wolf et al., 2007) (Table S1). Two recent reports have provided functional data indicating that phenotypic severity among ciliopathies is a function of mutational load. Our group identified new sequence variants in MKS-associated genes MKS1, MKS3, and CEP290 in our BBS cohort. Based on an in vivo zebrafish assay to assess the pathogenicity of the missense variants, hypomorphic alleles in MKS1 and MKS3 can contribute pathogenic alleles to the BBS phenotype (Leitch et al., 2008). Similarly, Bergmann and colleagues used ciliopathy patients and mouse models to demonstrate a phenotypic continuum between NPHP- and MKS-like phenotypes. In addition to their previous findings that NPHP3 mutations can cause the renal cystic phenotype of NPHP, they now report pathogenic NPHP3 mutations in fetuses with MKS-like phenotypes. They then modeled the pathogenic significance of these mutations by comparing the phenotypes of the pcy mouse, which harbors a hypomorphic mutation in Nphp3, with a mouse possessing a targeted null Nphp3 allele (Bergmann et al., 2008). Taken together, these studies provide evidence in favor of the ciliopathies as an organellar disorder, rather than multiple distinct clinical entities, the phenotype being a function (or a consequence) of total mutational load in ciliary protein-encoding genes.
The CEP290 gene product, a centrosomal protein expressed in renal epithelial cells and retinal photoreceptors, has emerged recently as a panciliopathy gene. Hypomorphic alleles in CEP290 represent the largest single contributor of mutations to Leber congenital amaurosis (LCA) patients, and a variant conferring only partial protein function is the underlying cause of the retinal phenotype in the rd16 mouse (Chang et al., 2006; den Hollander et al., 2006). CEP290 variants of largely uncharacterized pathogenic potential have also been associated with NPHP, JBTS, and BBS (Leitch et al., 2008; Sayer et al., 2006; Valente et al., 2006). Finally, on the severe end of the phenotypic spectrum, presumably null mutations introducing nonsense or frame-shifting mutations in the extreme N terminus of CEP290 have been reported in MKS fetuses (Baala et al., 2007a). Combined, these reports provide further evidence of allelic variation that is contributing to the phenotypic range among ciliopathies.
Emergence of Previously Unrecognized Ciliary Phenotypes
One of the challenges to ciliary disease is the difficulty in making an accurate patient diagnosis, considering the interplay of multiple genes, alleles, and phenotypes. Renal cystic disease coupled to polydactyly, vision loss, and/or central nervous system defects represent some classical hallmarks of ciliopathies when present in tandem. Two subtle pathogenic phenotypes have been reported recently that expand our understanding of ciliary function in particular cell types and will potentially contribute to the predictive and diagnostic power of ciliary phenotypes.
Vertebrate odorant sensation is mediated by the sensory cilia that decorate the olfactory sensory neurons. Through a still incompletely understood process, odorants bind to G-coupled receptors, and G proteins subsequently facilitate signal transduction in olfactory neurons (Buck and Axel, 1991). BBS patients, as well as mouse mutants lacking Bbs1 or Bbs4, manifest anosmia with concomitant organizational defects of the ciliated layer of the olfactory epithelium and disorganized microtubular structures in apical dendrites (Kulaga et al., 2004). Recently, this phenotype has been also observed in the rd16 mouse with partial loss of function of CEP290; electro-olfactograms indicated that the mice were anosmic, probably because of the mislocalization of olfactory G proteins in the absence of CEP290 (McEwen et al., 2007).
Basal body proteins have also recently been shown to be critical for proper function of the peripheral nervous system and compromised function results in defective thermo- and mechanosensation. BBS proteins localize to the basal bodies of ciliated dorsal root ganglion neurons in normal mice; however, Bbs-deficient mice, as well as BBS patients, do not respond to the same temperature and mechanical stimulus range as their wild-type counterparts. In the absence of a gross ciliary structural explanation for these sensory phenotypes, the mislocalization of the STOM3 and TRPV channel proteins in Bbs-deficient dorsal root ganglion neurons provides preliminary functional clues of the cellular basis of the phenotype (Tan et al., 2007). Further study of dorsal root ganglion development and support cells such as keratinocytes and glial cells will enable the dissection of this complex phenotype.
Integrating Ciliary Biology and Human Ciliopathies
The unifying organellar basis of the ciliopathies, informed by the dissection of cellular biology in vitro and in vivo, provides unique opportunities in understanding human disease. First, the phenotypic overlap shared across ciliopathies, particularly pleiotropic disorders, holds potential predictive power for syndromes for which the genetic and functional basis are still unknown. For example, postaxial polydactyly in ciliopathy patients is attributable likely to defects in Shh signal transduction, not only evidenced by the ciliary mutant phenotypes, but also by the observed phenocopy of humans with GLI3 mutations (Hill et al., 2007). Further, anosmia in BBS and LCA patients is probably due to defective cellular transport of signaling proteins and/or receptors in ciliated olfactory epithelia. Still, care must be taken in the interpretation of ciliary phenotypes in humans; multiple cellular abnormalities have the potential to manifest similar phenotypes such as the developmental defects in the central nervous system resulting from both Wnt and Shh signal disruption. As our understanding of ciliary function increases in different tissue types, we anticipate that new phenotypes, which may be used for diagnostic purposes, will become apparent.
The concept of a unified collection of disorders with a ciliary basis holds much potential for disease gene discovery. The ciliary proteome predicts that pathogenic variants in as many as 1000 different genes may contribute to the total mutational load of the ciliopathies (Gherman et al., 2006), with many of them having functions that are still unknown. The increased use of ultra-high throughput sequencing technologies and the development of physiologically relevant functional assays should enhance our rate of assignment to total mutational load in patients. We predict that this information will promote a shift from a clinical to a molecular diagnosis in which a collection of a few hypomorphic alleles result in mild phenotypes, and null alleles result in lethal phenotypes.
Concluding Remarks
The vertebrate cilium is a sensory organelle critical for a broad array of homeostatic mechanisms and paracrine signals. Notably, ciliopathy phenotypes manifest as both developmental structural defects and failure of left-right axis determination in a host of clinical disorders, as well as progressive degenerative phenotypes, suggesting that ciliary proteins serve important functions in organogenesis, tissue maintenance, and, potentially, regeneration. The field faces numerous challenges for the future, which include three major questions.
First, what are the similarities and differences (both structural and functional) between primary cilia in different cell types? Given the association of major developmental process with ciliary dysfunction, it is perhaps paradoxical that ciliopathies do not manifest more severe clinical phenotypes. In addition to the usual suspicions of differential tolerance for damage and regenerative ability, it is likely that not all cilia partake in the same signaling processes. To that end, the progress made toward characterizing the complete ciliary proteome must now be refocused toward understanding the protein complement of cilia in particular tissues and cell types.
Second, it is equally important to differentiate between the ciliary and nonciliary roles of these proteins. There is a pervasive ciliocentric view in the current literature—that a protein localizes to basal bodies and cilia in cultured cells and tissues can no longer serve as definitive evidence that a protein’s function is exclusively at the cilium. Several ciliary proteins are now known to serve multiple cellular functions, and it is likely that pools of ciliary proteins will be found in other compartments under specific physiological conditions. Therefore, the burden of proof for a ciliary phenotype in any model system will require multiple independent lines of evidence, including studies of ciliation and deciliation.
Third, the association of the primary cilium with Hedgehog and Wnt signaling opens up several areas of investigation. Most pressing is to identify other pathways in which the primary cilium might play a role. Intriguingly, if basal body integrity is indeed required for proteasomal targeting of a subset of signaling proteins, including, but not limited to, β-catenin (Gerdes et al., 2007), we might expect pathways that require proteasomal processing to be modulated by the cilium. It is unlikely to be coincidental that both Hedgehog (Gli processing) and Wnt signaling (β-catenin degradation) rely heavily on proteasomal processing, and that disruption of both the basal body and cilium result in impaired proteasomal processing of Gli2/3 (Caspary et al., 2007; Delous et al., 2007; Haycraft et al., 2005; Huangfu and Anderson, 2005; Karmous-Benailly et al., 2005) and impaired degradation of Dvl and β-catenin (Gerdes et al., 2007; Simons et al., 2005).
Finally, the near-ubiquitous presence of ciliated structures in vertebrates potentially contains some important evolutionary lessons. In invertebrates, such as Drosophila, only sensory neurons and sperm are ciliated, whereas in vertebrates, nearly every tissue type has cells that are ciliated at some point in their life cycle. We suggest that the role of the cilium in paracrine signaling might have been the direct outcome of the widespread presence of cilia in vertebrates. Clearly, though conserved throughout evolution, signal transduction pathways have diverged from invertebrate to vertebrate organisms. In Drosophila, the Hedgehog and PCP signaling pathways are organized differently than they are in mice and humans. However, it is unclear which fraction of a specific signaling pathway is regulated by the primary cilium, though such regulation is likely to be time and tissue specific.
Supplementary Material
Acknowledgments
We are indebted to J. Badano, E. Oh, J. Robinson, and N. Zaghloul for their critical review of the manuscript and A. Gherman for technical assistance. This work was supported by grant R01HD04260 from the National Institute of Child Health and Development (N.K.), R01DK072301, and R01DK075972 (N.K.) and NRSA fellowship F32 DK079541 (E.E.D.) from the National Institute of Diabetes, Digestive and Kidney Disorders, the Macular Vision Research Foundation (N.K.), and the Foundation for Fighting Blindness (N.K.).
Footnotes
Supplemental Data include one Table and can be found with this article online at http://www.cell.com/supplemental/S0092-8674(09)00322-5.
References
- Abdelkhalek HB, Beckers A, Schuster-Gossler K, Pavlova MN, Burkhardt H, Lickert H, Rossant J, Reinhardt R, Schalkwyk LC, Müller I, et al. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004;18:1725–1736. doi: 10.1101/gad.303504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–888. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, Saunier S, Salomon R, Gonzales M, Rattenberry E, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007a;81:170–179. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007b;80:186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left-right patterning. Proc Natl Acad Sci USA. 2007;104:15675–15770. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl Syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLoungis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, et al. The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Biol Cell. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisogueil M, Miquel M, Hamon M, Verge D. Localization of 5-HT6 receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD. Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol. 2000;23:45–51. doi: 10.1165/ajrcmb.23.1.4070. [DOI] [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Knowles H, Hebert J, Hackett B. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102:1066–1072. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter J. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, Voesenek KE, Zonneveld MN, Strom TM, Meitinger T, Brunner HG, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 1995;11:398–404. doi: 10.1016/s0168-9525(00)89123-4. [DOI] [PubMed] [Google Scholar]
- Efimenko E, Bubb K, Mak HY, Holzman T, Leroux MR, Ruvkun G, Thomas JH, Swoboda P. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–1934. doi: 10.1242/dev.01775. [DOI] [PubMed] [Google Scholar]
- Eley L, Turnpenny L, Yates LM, Craighead AS, Morgan D, Whistler C, Goodship JA, Strachan T. A perspective on inversin. Cell Biol Int. 2004;28:119–124. doi: 10.1016/j.cellbi.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Fan S, Hurd T, Liu C, Straight S, Weimbs T, Hurd E, Domino S, Margolis B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Feistel K, Blum M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn. 2006;235:3348–3358. doi: 10.1002/dvdy.20986. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz S, Giddings TH, Jr, Gomez-Ospina N, Dutcher SK. J Cell Sci. 2004;117:303–314. doi: 10.1242/jcs.00859. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson S, Kato M, Beachy P, Beales P, DeMartino G, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Singaraja R, Xanthoudakis S, Gutekunst CA, Leavitt BR, Metzler M, Hackam AS, Tam J, Vaillancourt JP, Houtzager V, et al. Recruitment and activation of caspase-8 by the Huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat Cell Biol. 2002;4:95–105. doi: 10.1038/ncb735. [DOI] [PubMed] [Google Scholar]
- Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Haraguchi K, Hayashi T, Jimbo T, Yamamoto T, Akiyama T. Role of the kinesin-2 family protein, KIF3, during mitosis. J Biol Chem. 2006;281:4094–4099. doi: 10.1074/jbc.M507028200. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:480–488. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134:307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Hill P, Wang B, Ruther U. The molecular basis of Pallister Hall associated polydactyly. Hum Mol Genet. 2007;16:2089–2096. doi: 10.1093/hmg/ddm156. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Houde C, Dickinson R, Houtzager V, Cullum R, Montpetit R, Metzler M, Simpson E, Roy S, Hayden M, Hoodless P, Nicholson D. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300:523–533. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Karmous-Benailly H, Martinovic J, Gubler MC, Sirot Y, Clech L, Ozilou C, Auge J, Brahimi N, Etchevers H, Detrait E, et al. Antenatal presentation of Bardet-Biedl syndrome may mimic Meckel syndrome. Am J Hum Genet. 2005;76:493–504. doi: 10.1086/428679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller LC, Geimer S, Romijn E, Yates J, 3rd, Zamora I, Marshall WF. Molecular architecture of the centriole proteome: the conserved WD40 domain protein POC1 is required for centriole duplication and length control. Mol Biol Cell. 2008;20:1150–1166. doi: 10.1091/mbc.E08-06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, Maeda Y, Lanske B, Song B, Serra R, Pacifici M. Conditional Kif3a ablation causes abnormal hedgehog siganling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134:2159–2169. doi: 10.1242/dev.001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36:994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Le Bot N, Antony C, White J, Karsenti E, Vernos I. Role of Xklp3, a Subunit of the Xenopus Kinesin II Heterotrimeric Complex, in Membrane Transport betwewen the Endoplasmic Reticulum and the Golgi Apparatus. J Cell Biol. 1998;143:1559–1573. doi: 10.1083/jcb.143.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Al-Fadhel M, Lewis RA, Eyaid W, Banin E, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomic identification of conserved flagellar and basal body proteins that includes a novel gene for Bardet-Biedl syndrome. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair A, Goldstein L, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander L. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007a;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lu W, Obara T, Kuida S, Lehoczky J, Dewar K, Drummond I, Beier D. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–5846. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the ifferentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007b;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- Lum L, Beachy P. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Ma M, Jiang YJ. Jagged2a-notch signaling mediates cell fate choice in the zebrafish pronephric duct. PLoS Genet. 2007;3:e18. doi: 10.1371/journal.pgen.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoub MR, Trapp ML, Quarmby LM. NIMA-related kinases defective in murine models of polycystic kidney diseases localize to primary cilia and centrosomes. J Am Soc Nephrol. 2005;16:3485–3489. doi: 10.1681/ASN.2005080824. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Curr Opin Cell Biol. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287:378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, Martens JR. Hypomorphic CEP290/NPHP6 mutations result in anosmia caused by the selective loss of G proteins in cilia of olfactory sensory neurons. Proc Natl Acad Sci USA. 2007;104:15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1203. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kato K, Yamaguchi T, Fukata Y, Ohno S, Kaibuchi K. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat Cell Biol. 2004;6:328–334. doi: 10.1038/ncb1118. [DOI] [PubMed] [Google Scholar]
- Nurnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell. 2002;13:3096–3106. doi: 10.1091/mbc.E02-04-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Krien MJ, Hunter T. Never say never. The NI-MA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13:221–228. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Bemonte JI. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Pan J, Wang Q, Snell W. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev Cell. 2004;6:445–451. doi: 10.1016/s1534-5807(04)00064-4. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Poole CA, Jensen CG, Snyder JA, Gray CG, Hermanutz VL, Wheatley DN. Confocal Analysis of primary cilia structures and colocalization with the Golgi Apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol Int. 1997;21:483–494. doi: 10.1006/cbir.1997.0177. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC, Torres VE. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- Reese TS. Olfactory cilia in the frog. J Cell Biol. 1965;25:209–230. doi: 10.1083/jcb.25.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott M. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KWY, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ. The ins and outs of Wingless signaling. Trends Cell Biol. 2004;14:45–53. doi: 10.1016/j.tcb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3beta are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1964;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Han Y, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo J, Alvarez-Buylla A. Primary cilia are required for cerebrellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spektor A, Tsang W, Khoo D, Dynlacht B. Cep97 and CP110 Suppress a Cilia Assembly Program. Cell. 2007;130:678–690. doi: 10.1016/j.cell.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Stubbs JL, Oishi I, Izpisua Belmonte JC, Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ. The key to left-right asymmetry. Cell. 2006;127:27–32. doi: 10.1016/j.cell.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Tan PL, Barr T, Inglis PN, Mitsuma N, Huang SM, Garcia-Gonzalez MA, Bradley BA, Coforio S, Albrecht PJ, Watnick T, et al. Loss of Bardet Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proc Natl Acad Sci USA. 2007;104:17524–17529. doi: 10.1073/pnas.0706618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci. 2008;121:1193–1203. doi: 10.1242/jcs.015495. [DOI] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV, et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet. 2008;40:403–410. doi: 10.1038/ng.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal. 2007;19:444–453. doi: 10.1016/j.cellsig.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya P, Birkenmeier E, Birkenmeier C, Barker J. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA. 2000;97:217–221. doi: 10.1073/pnas.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten J, Dildrop R, Peters T, Wang B, Ruther U. Ftm is a novel basal body protein of cilia invilved in Shh signaling. Development. 2007;134:2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol. 2004;15:592–602. doi: 10.1097/01.asn.0000113793.12558.1d. [DOI] [PubMed] [Google Scholar]
- Wolf MT, Saunier S, O’Toole JF, Wanner N, Groshong T, Attanasio M, Salomon R, Stallmach T, Sayer JA, Waldherr R, et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–1388. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- Zhang M, Bolfing M, Knowles H, Karnes H, Hackett B. Foxj1 regulates asymmetric gene expression during left-right axis patterning in mice. Biochem Biophys Res Commun. 2004;324:1413–1420. doi: 10.1016/j.bbrc.2004.09.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.